Sideritis Clandestina (Bory & Chaub.) Hayek; Sideritis Raeseri Boiss
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity
applied sciences Article The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity Małgorzata Ziarno 1,* , Mariola Kozłowska 2 , Iwona Scibisz´ 3 , Mariusz Kowalczyk 4 , Sylwia Pawelec 4 , Anna Stochmal 4 and Bartłomiej Szleszy ´nski 5 1 Division of Milk Technology, Department of Food Technology and Assessment, Institute of Food Science, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland 2 Department of Chemistry, Institute of Food Science, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland; [email protected] 3 Division of Fruit, Vegetable and Cereal Technology, Department of Food Technology and Assessment, Institute of Food Science, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland; [email protected] 4 Department of Biochemistry and Crop Quality, Institute of Soil Science and Plant Cultivation, State Research Institute, 24-100 Puławy, Poland; [email protected] (M.K.); [email protected] (S.P.); [email protected] (A.S.) 5 Institute of Horticultural Sciences, Warsaw University of Life Sciences–SGGW (WULS–SGGW), 02-787 Warsaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-225-937-666 Abstract: This study aimed to investigate the effect of plant extracts (valerian Valeriana officinalis L., sage Salvia officinalis L., chamomile Matricaria chamomilla L., cistus Cistus L., linden blossom Tilia L., ribwort plantain Plantago lanceolata L., marshmallow Althaea L.) on the activity and growth of lactic acid bacteria (LAB) during the fermentation and passage of milk through a digestive system model. Citation: Ziarno, M.; Kozłowska, M.; The tested extracts were also characterized in terms of their content of polyphenolic compounds and Scibisz,´ I.; Kowalczyk, M.; Pawelec, S.; antioxidant activity. -

Essential Oil Composition of Two Greek Cultivated Sideritis Spp
Nat. Volatiles & Essent. Oils, 2019; 6(3): 16-23 Kloukina et al. RESEARCH ARTICLE Essential oil composition of two Greek cultivated Sideritis spp. Charalampia Kloukina, Ekaterina-Michaela Tomou, Helen Skaltsa* Department of Pharmacognosy & Chemistry of Natural Products, School of Pharmacy, National and Kapodistrian University of Athens, Athens, Greece *Corresponding author. Email: [email protected] Abstract In this present work, essential oil composition of cultivated commercially available Sideritis raeseri Boiss. & Heldr. and Sideritis scardica Griseb. from three different restricted areas of Kozani (central Greece) were studied. The essential oils were obtained by hydrodistillation in a modified Clevenger-type apparatus, and their analyses were performed by GC-MS. Monoterpene hydrocarbons constituted the main fraction of S. raeseri from Polirraxo (Kozani) and of S. scardica from Chromio (Kozani), while sesquiterpene hydrocarbons were the main group of S. raeseri from Metamorfosis (Kozani) and S. scardica from Metamorfosis (Kozani). Despite the different cultivations, some constituents were found even in different percentages in both samples of S. scardica: α-pinene, -pinene, cis-caryophyllene, bicyclogermacrene and germacrene D. It is noteworthy that the two samples of S. raeseri have totally different main constituents, which could be related to the different cultivation conditions, as well as to the known tendency of some Sideritis species to hybridize, which suggests further research. Keywords: Sideritis raeseri, Sideritis scardica, cultivation, composition of volatiles Introduction Sideritis L. (Lamiaceae) comprises more than 150 species, indigenous in Central Europe, the Mediterranean, the Balkans, the Iberian Peninsula and the west Asia (González-Burgos et al., 2011). Traditionally, the infusion of herba Sideritis (S. scardica, S. -

Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families Against in Vitro Human Tumor Models
ANTICANCER RESEARCH 27: 3293-3300 (2007) Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families Against In Vitro Human Tumor Models MONICA ROSA LOIZZO1, ROSA TUNDIS1, FEDERICA MENICHINI1, ANTOINE MIKAEL SAAB2, GIANCARLO ANTONIO STATTI1 and FRANCESCO MENICHINI1 1Faculty of Pharmacy, Nutrition and Health Sciences, Department of Pharmaceutical Sciences, University of Calabria, I-87036 Rende (CS), Italy; 2Faculty of Sciences II, Chemistry Department, Lebanese University, P.O. Box :90656 Fanar, Beirut, Lebanon Abstract. Background: The aim of this work was to study undertaken on the cytotoxic activity of essential oils from the cytotoxicity of essential oils and their identified Sideritis perfoliata, Satureia thymbra, Salvia officinalis, Laurus constituents from Sideritis perfoliata, Satureia thymbra, nobilis or Pistacia palestina. Salvia officinalis, Laurus nobilis and Pistacia palestina. The genus Sideritis (Labiatae) is of great botanical and Materials and Methods: Essential oils were obtained by pharmacological interest, in fact many species are reported hydrodistillation and were analysed by gas chromatography to have analgesic, anti-inflammatory, antibacterial, (GC) and GC/mass spectrometry (MS). The cytotoxic activity antirheumatic, anti-ulcer, digestive and vaso-protective was evaluated in amelanotic melanoma C32, renal cell properties and have been used in Mediterranean folk adenocarcinoma ACHN, hormone-dependent prostate medicine (11). No reports have been found concerning the carcinoma LNCaP, and MCF-7 breast cancer cell lines by phytochemical composition or biological or cytotoxic activity the sulforhodamine B (SRB) assay. Results: L. nobilis fruit of S. perfoliata (12). S. thymbra (Labiatae) is the most oil exerted the highest activity with IC50 values on C32 and common Satureja specimen and is known as a herbal home ACHN of 75.45 and 78.24 Ìg/ml, respectively. -

Shilin Yang Doctor of Philosophy
PHYTOCHEMICAL STUDIES OF ARTEMISIA ANNUA L. THESIS Presented by SHILIN YANG For the Degree of DOCTOR OF PHILOSOPHY of the UNIVERSITY OF LONDON DEPARTMENT OF PHARMACOGNOSY THE SCHOOL OF PHARMACY THE UNIVERSITY OF LONDON BRUNSWICK SQUARE, LONDON WC1N 1AX ProQuest Number: U063742 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U063742 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENT I wish to express my sincere gratitude to Professor J.D. Phillipson and Dr. M.J.O’Neill for their supervision throughout the course of studies. I would especially like to thank Dr. M.F.Roberts for her great help. I like to thank Dr. K.C.S.C.Liu and B.C.Homeyer for their great help. My sincere thanks to Mrs.J.B.Hallsworth for her help. I am very grateful to the staff of the MS Spectroscopy Unit and NMR Unit of the School of Pharmacy, and the staff of the NMR Unit, King’s College, University of London, for running the MS and NMR spectra. -

Phenolic Profiling of Veronica Spp. Grown in Mountain, Urban and Sand Soil Environments
CORE Metadata, citation and similar papers at core.ac.uk Provided by Biblioteca Digital do IPB Phenolic profiling of Veronica spp. grown in mountain, urban and sand soil environments João C.M. Barreiraa,b,c, Maria Inês Diasa,c, Jelena Živkovićd, Dejan Stojkoviće, Marina Sokoviće, Celestino Santos-Buelgab,*, Isabel C.F.R. Ferreiraa,* aCIMO/Escola Superior Agrária, Instituto Politécnico de Bragança, Apartado 1172, 5301-855 Bragança, Portugal. bGIP-USAL, Facultad de Farmacia, Universidad de Salamanca, Campus Miguel de Unamuno, 37007 Salamanca, Spain. cREQUIMTE/Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, nº 228, 4050-313 Porto, Portugal. dInstitute for Medicinal Plant Research “Dr. Josif Pančić”, Tadeuša Košćuška 1, 11000 Belgrade, Serbia. eDepartment of Plant Physiology, Institute for Biological Research “Siniša Stanković”, University of Belgrade, Bulevar Despota Stefana 142, 11000 Belgrade, Serbia. * Authors to whom correspondence should be addressed (Isabel C.F.R. Ferreira; e-mail: [email protected], telephone +351273303219, fax +351273325405; e-mail: Celestino Santos- Buelga: [email protected]; telephone +34923294537; fax +34923294515). 1 Abstract Veronica (Plantaginaceae) genus is widely distributed in different habitats. Phytochemistry studies are increasing because most metabolites with pharmacological interest are obtained from plants. The phenolic compounds of V. montana, V. polita and V. spuria were tentatively identified by HPLC-DAD-ESI/MS. The phenolic profiles showed that flavones were the major compounds (V. montana: 7 phenolic acids, 5 flavones, 4 phenylethanoids and 1 isoflavone; V. polita: 10 flavones, 5 phenolic acids, 2 phenylethanoids, 1 flavonol and 1 isoflavone; V. spuria: 10 phenolic acids, 5 flavones, 2 flavonols, 2 phenylethanoids and 1 isoflavone), despite the overall predominance of flavones. -

Herbal Principles in Cosmetics Properties and Mechanisms of Action Traditional Herbal Medicines for Modern Times
Traditional Herbal Medicines for Modern Times Herbal Principles in Cosmetics Properties and Mechanisms of Action Traditional Herbal Medicines for Modern Times Each volume in this series provides academia, health sciences, and the herbal medicines industry with in-depth coverage of the herbal remedies for infectious diseases, certain medical conditions, or the plant medicines of a particular country. Series Editor: Dr. Roland Hardman Volume 1 Shengmai San, edited by Kam-Ming Ko Volume 2 Rasayana: Ayurvedic Herbs for Rejuvenation and Longevity, by H.S. Puri Volume 3 Sho-Saiko-To: (Xiao-Chai-Hu-Tang) Scientific Evaluation and Clinical Applications, by Yukio Ogihara and Masaki Aburada Volume 4 Traditional Medicinal Plants and Malaria, edited by Merlin Willcox, Gerard Bodeker, and Philippe Rasoanaivo Volume 5 Juzen-taiho-to (Shi-Quan-Da-Bu-Tang): Scientific Evaluation and Clinical Applications, edited by Haruki Yamada and Ikuo Saiki Volume 6 Traditional Medicines for Modern Times: Antidiabetic Plants, edited by Amala Soumyanath Volume 7 Bupleurum Species: Scientific Evaluation and Clinical Applications, edited by Sheng-Li Pan Traditional Herbal Medicines for Modern Times Herbal Principles in Cosmetics Properties and Mechanisms of Action Bruno Burlando, Luisella Verotta, Laura Cornara, and Elisa Bottini-Massa Cover art design by Carlo Del Vecchio. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2010 by Taylor and Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-13: 978-1-4398-1214-3 (Ebook-PDF) This book contains information obtained from authentic and highly regarded sources. -

Resilience at the Border: Traditional Botanical Knowledge Among Macedonians and Albanians Living in Gollobordo, Eastern Albania
Pieroni et al. Journal of Ethnobiology and Ethnomedicine 2014, 10:31 http://www.ethnobiomed.com/content/10/1/31 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access Resilience at the border: traditional botanical knowledge among Macedonians and Albanians living in Gollobordo, Eastern Albania Andrea Pieroni1*, Kevin Cianfaglione2, Anely Nedelcheva3, Avni Hajdari4, Behxhet Mustafa4 and Cassandra L Quave5,6 Abstract Background: Ethnobotany in South-Eastern Europe is gaining the interest of several scholars and stakeholders, since it is increasingly considered a key point for the re-evaluation of local bio-cultural heritage. The region of Gollobordo, located in Eastern Albania and bordering the Republic of Macedonia, is of particular interest for conducting ethnobiological studies, since it remained relatively isolated for the larger part of the 20th Century and is traditionally inhabited by a majority of ethnic Macedonians and a minority of Albanians (nowadays both sharing the Muslim faith). Methods: An ethnobotanical survey focused on local food, medicinal, and veterinary plant uses was conducted with 58 participants using open and semi-structured interviews and via participant observation. Results: We recorded and identified 115 taxa of vascular plants, which are locally used for food, medicinal, and veterinary purposes (representing 268 total plant reports). The Macedonian Traditional Ecological Knowledge (TEK) was greater than the Albanian TEK, especially in the herbal and ritual domains. This phenomenon may be linked to the long socio-cultural and linguistic isolation of this group during the time when the borders between Albania and the former Yugoslavia were completely closed. Moreover, the unusual current food utilisation of cooked potatoes leaves, still in use nowadays among Macedonians, could represent the side effect of an extreme adaptation that locals underwent over the past century when the introduction of the potato crop made new strategies available for establishing stable settlements around the highest pastures. -

Cushnie TPT, Lamb AJ. Antimicrobial Activity of Flavonoids. International Journal of Antimicrobial Agents, 2005. 26(5):343-356
Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 2005. 26(5):343-356. PMID: 16323269 DOI: 10.1016/j.ijantimicag.2005.09.002 The journal article above is freely available from the publishers at: http://www.idpublications.com/journals/PDFs/IJAA/ANTAGE_MostCited_1.pdf and also... http://www.ijaaonline.com/article/S0924-8579(05)00255-4/fulltext Errata for the article (typesetting errors by Elsevier Ireland) are freely available from the publishers at: http://www.ijaaonline.com/article/S0924-8579(05)00352-3/fulltext and also... http://www.sciencedirect.com/science/article/pii/S0924857905003523 International Journal of Antimicrobial Agents 26 (2005) 343–356 Review Antimicrobial activity of flavonoids T.P. Tim Cushnie, Andrew J. Lamb ∗ School of Pharmacy, The Robert Gordon University, Schoolhill, Aberdeen AB10 1FR, UK Abstract Flavonoids are ubiquitous in photosynthesising cells and are commonly found in fruit, vegetables, nuts, seeds, stems, flowers, tea, wine, propolis and honey. For centuries, preparations containing these compounds as the principal physiologically active constituents have been used to treat human diseases. Increasingly, this class of natural products is becoming the subject of anti-infective research, and many groups have isolated and identified the structures of flavonoids possessing antifungal, antiviral and antibacterial activity. Moreover, several groups have demonstrated synergy between active flavonoids as well as between flavonoids and existing chemotherapeutics. Reports of activity in the field of antibacterial flavonoid research are widely conflicting, probably owing to inter- and intra-assay variation in susceptibility testing. However, several high-quality investigations have examined the relationship between flavonoid structure and antibacterial activity and these are in close agreement. -

Distribution of Flavonoids Among Malvaceae Family Members – a Review
Distribution of flavonoids among Malvaceae family members – A review Vellingiri Vadivel, Sridharan Sriram, Pemaiah Brindha Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University, Thanjavur, Tamil Nadu, India Abstract Since ancient times, Malvaceae family plant members are distributed worldwide and have been used as a folk remedy for the treatment of skin diseases, as an antifertility agent, antiseptic, and carminative. Some compounds isolated from Malvaceae members such as flavonoids, phenolic acids, and polysaccharides are considered responsible for these activities. Although the flavonoid profiles of several Malvaceae family members are REVIEW REVIEW ARTICLE investigated, the information is scattered. To understand the chemical variability and chemotaxonomic relationship among Malvaceae family members summation of their phytochemical nature is essential. Hence, this review aims to summarize the distribution of flavonoids in species of genera namely Abelmoschus, Abroma, Abutilon, Bombax, Duboscia, Gossypium, Hibiscus, Helicteres, Herissantia, Kitaibelia, Lavatera, Malva, Pavonia, Sida, Theobroma, and Thespesia, Urena, In general, flavonols are represented by glycosides of quercetin, kaempferol, myricetin, herbacetin, gossypetin, and hibiscetin. However, flavonols and flavones with additional OH groups at the C-8 A ring and/or the C-5′ B ring positions are characteristic of this family, demonstrating chemotaxonomic significance. Key words: Flavones, flavonoids, flavonols, glycosides, Malvaceae, phytochemicals INTRODUCTION connate at least at their bases, but often forming a tube around the pistils. The pistils are composed of two to many connate he Malvaceae is a family of flowering carpels. The ovary is superior, with axial placentation, with plants estimated to contain 243 genera capitate or lobed stigma. The flowers have nectaries made with more than 4225 species. -

Serbian Journal of Experimental and Clinical Research Vol12
Editor-in-Chief Slobodan Janković Co-Editors Nebojša Arsenijević, Miodrag Lukić, Miodrag Stojković, Milovan Matović, Slobodan Arsenijević, Nedeljko Manojlović, Vladimir Jakovljević, Mirjana Vukićević Board of Editors Ljiljana Vučković-Dekić, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia Dragić Banković, Faculty for Natural Sciences and Mathematics, University of Kragujevac, Kragujevac, Serbia Zoran Stošić, Medical Faculty, University of Novi Sad, Novi Sad, Serbia Petar Vuleković, Medical Faculty, University of Novi Sad, Novi Sad, Serbia Philip Grammaticos, Professor Emeritus of Nuclear Medicine, Ermou 51, 546 23, Th essaloniki, Macedonia, Greece Stanislav Dubnička, Inst. of Physics Slovak Acad. Of Sci., Dubravska cesta 9, SK-84511 Bratislava, Slovak Republic Luca Rosi, SAC Istituto Superiore di Sanita, Vaile Regina Elena 299-00161 Roma, Italy Richard Gryglewski, Jagiellonian University, Department of Pharmacology, Krakow, Poland Lawrence Tierney, Jr, MD, VA Medical Center San Francisco, CA, USA Pravin J. Gupta, MD, D/9, Laxminagar, Nagpur – 440022 India Winfried Neuhuber, Medical Faculty, University of Erlangen, Nuremberg, Germany Editorial Staff Ivan Jovanović, Gordana Radosavljević, Nemanja Zdravković Vladislav Volarević Management Team Snezana Ivezic, Milan Milojevic, Bojana Radojevic, Ana Miloradovic Corrected by Scientifi c Editing Service “American Journal Experts” Design PrstJezikIostaliPsi - Miljan Nedeljković Print Medical Faculty, Kragujevac Indexed in EMBASE/Excerpta Medica, Index Copernicus, BioMedWorld, KoBSON, -
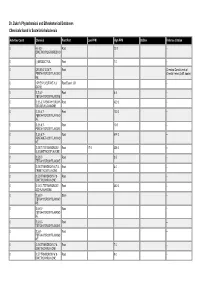
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals Found in Scutellaria Baicalensis
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals found in Scutellaria baicalensis Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 (+)-5,5'- Root 22.0 -- DIMETHOXYLARICIRESINO L 0 (-)-ERODICTYOL Root 7.0 -- 0 (2R,3R)-2',3,5,6',7- Root Chemical Constituents of PENTAHYDROXYFLAVONO Oriental Herbs (3 diff. books) NE 0 1-PHENYL-BUTANE-1,3- Root Essent. Oil -- DIONE 0 2',3',5,7- Root 6.4 -- TETRAHYDROXYFLAVONE 0 2',3,5,6',7-PENTAHYDROXY- Root 420.0 -- 2(R),3(R)-FLAVANONE 0 2',3,5,6',7- Root 120.0 -- PENTAHYDROXYFLAVANO NE 0 2',3,5,6',7- Root 10.0 -- PENTAHYDROXYFLAVONE 0 2',3,5,6',7- Root 674.0 -- PENTAMETHOXYFLAVANO NE 0 2',5,5',7-TETRAHYDROXY- Root 17.0 356.0 -- 6',8-DIMETHOXYFLAVONE 0 2',5,5',7- Root 3.5 -- TETRAHYDROXYFLAVONE 0 2',5,5'-TRIHYDROXY-6,7,8- Root 4.0 -- TRIMETHOXYFLAVONE 0 2',5,5'-TRIHYDROXY-7,8- Root -- DIMETHOXYFLAVONE 0 2',5,6',7-TETRAHYDROXY- Root 380.0 -- 2(S)-FLAVANONE 0 2',5,6',7- Stem -- TETRAHYDROXYFLAVANO NE 0 2',5,6',7- Root -- TETRAHYDROXYFLAVANO NE 0 2',5,6',7- Root -- TETRAHYDROXYFLAVONE 0 2',5,6'- Root -- TETRAHYDROXYFLAVANO NE 0 2',5,6'-TRIHYDROXY-7,8- Root 7.0 -- DIMETHOXYFLAVONE 0 2',5,7-TRIHYDROXY-6',8- Root 9.0 -- DIMETHOXYFLAVONE Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 2',5,7-TRIHYDROXY-6',8- Root 7.0 -- TRIMETHOXYFLAVONE 0 2',5,7-TRIHYDROXY-6'- Root 4.0 -- METHOXYFLAVONE 0 2',5,7-TRIHYDROXY-8- Root 6.0 -- METHOXYFLAVONE 0 2',5,7- Root 6.0 -- TRIHYDROXYFLAVONE 0 2',5,8-TRIHYDROXY-6,7- Root 200.0 -- DIMETHOXYFLAVONE 0 2'- Root -- METHYLSKULLCAPFLAVO NE 0 2(S),2',5,6',7- Root 230.0 -- TETRAHYDROXYFLAVANO NE 0 2,2',4',6-TETRAHYDROXY- Root 5.0 -- 6'-METHOXYCHALCONE 1 2,3-DIHYDROBENZOFURAN Root Essent. -

Sideritis Perfoliata (Subsp
antioxidants Article Sideritis Perfoliata (Subsp. Perfoliata) Nutritive Value and Its Potential Medicinal Properties Namrita Lall 1,2,3,*, Antonios Chrysargyris 4, Isa Lambrechts 1, Bianca Fibrich 1, Analike Blom Van Staden 1, Danielle Twilley 1, Marco Nuno de Canha 1, Carel Basson Oosthuizen 1 , Dikonketso Bodiba 1 and Nikolaos Tzortzakis 4,* 1 Department of Plant and Soil Sciences, University of Pretoria, Pretoria 0002, South Africa; [email protected] (I.L.); bianca.fi[email protected] (B.F.); [email protected] (A.B.V.S.); [email protected] (D.T.); [email protected] (M.N.d.C.); [email protected] (C.B.O.); [email protected] (D.B.) 2 School of Natural Resources, University of Missouri, Columbia, MO 65211, USA 3 College of Pharmacy, JSS Academy of Higher Education and Research, Mysuru, Karnataka 570015, India 4 Department of Agricultural Sciences, Biotechnology and Food Science, Cyprus University of Technology, 3036 Lemesos, Cyprus; [email protected] * Correspondence: [email protected] (N.L.); [email protected] (N.T.); Tel.: +27-124206670 (N.L.); +357-25002280 (N.T.) Received: 11 September 2019; Accepted: 25 October 2019; Published: 30 October 2019 Abstract: Sideritis perfoliata L. subsp. perfoliata is an endemic species of the Eastern Mediterranean region with several uses in traditional medicine. The present study aims to explore the unknown properties of S. perfoliata investigating the nutritional content as well as the antioxidant, anticancer, antituberculosis, antiwrinkle, anti-acne, hyper/hypo-pigmentation and antibacterial activities. Mineral content, nutritional value, the composition and antioxidant properties of the essential oil, the antityrosinase, the antibacterial activity and anti-elastase potential of the extract, were evaluated.