Cytotoxic Activity of Essential Oils from Labiatae and Lauraceae Families Against in Vitro Human Tumor Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

12 Great Ways to Use Fennel
12 Great Ways to Use… Fennel Fennel’s fragrant anise-like flavor pairs nicely with onions, garlic, lemon, fresh herbs, seafood, chicken, and pork. Fennel can be sautéed, braised, roasted, baked, or enjoyed raw. And because the bulb, the fronds, and even fennel seeds are all deliciously edible, it is a wildly versatile ingredient. One cup of raw, sliced fennel bulb has less than 30 calories but offers 3g of fiber and more than 15% of the recommended daily intake of vitamin C. Make a risotto. Start by sautéing chopped Toss fennel wedges and chopped winter 1. fennel bulbs and stalks, onions, and garlic, 9. squash or root vegetables (like carrots, then add the rice and cooking liquid. parsnips, or beets) with olive oil, salt and pepper. Roast at 400⁰F for about 30 Caramelize slices of fennel and onion minutes (tossing once half-way through) or 2. together. Use the caramelized veggies for a until fork tender. sandwich or pizza topping. Make fennel tea by adding 2 teaspoons of Add thinly sliced fennel and chopped 10. crushed fennel seeds or a small bunch of 3. walnuts to a coleslaw or cabbage salad. coarsely chopped fennel fronds to 2 cups of boiling water. Steep for 5-10 minutes for Combine sliced fennel, celery, radish, and tea made with seeds or for 3-5 minutes for 4. arugula into a salad and dress with a lemon tea made with fronds. vinaigrette. Fold feta cheese and sautéed fennel into Layer the bottom of a baking dish with 11. wilted spinach for an easy side dish. -

Pdf 550.58 K
Iranian Journal of Pharmaceutical Research (2014),13 (supplement): 195-198 Copyright © 2014 by School of Pharmacy Received: December 2013 Shaheed Beheshti University of Medical Sciences and Health Services Accepted: December 2013 Original Article Screening of 20 Commonly Used Iranian Traditional Medicinal Plants Against Urease Mahmood Biglara, Hessameddin Sufia, Kowsar Bagherzadeha, Massoud Amanloua and Faraz Mojabb* aDepartment of Medicinal Chemistry, Faculty of Pharmacy and Pharmaceutical Science Research Center, Tehran University of Medical Sciences, Tehran, Iran. bDepartment of Pharmacognosy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. Abstract Infection with Helicobacter pyloriis the most common cause of stomach and duodenal ulcers. About more than 80 % of people are infected with H. pylori in developing countries. H. pylori uses urease enzyme product “ammonia” in order to neutralize and protect itself from the stomach acidic condition and urease enzyme activity has been shown to be essential to the colonization of H. pylori. Inhibitory activity of 20 traditional medicinal plants were examined and evaluated against Jack bean urease activity by Berthelot reaction to obtains natural sources of urease inhibitors. Each herb was extracted using 80% aqueous methanol, then tested its IC50 value was determined. Eight of the whole 20 studied plants crude extracts were found the most effective with IC50 values of less than 100 µg/mL including Laurus nobilis, Zingiber officinale, Nigella sativa, Angelica archangelica, Acorus calamus, Allium sativum,Curcuma longa, and Citrus aurantium extracts, from which most potent urease inhibitory was observed for Zingiber officinale, Laurus nobilis, and Nigella sativa with IC50 values of 48.54, 48.69 and 59.10 µg/mL, respectively. -

Fragrant Herbs for Your Garden
6137 Pleasants Valley Road Vacaville, CA 95688 Phone (707) 451-9406 HYPERLINK "http://www.morningsunherbfarm.com" www.morningsunherbfarm.com HYPERLINK "mailto:[email protected]" [email protected] Fragrant Herbs For Your Garden Ocimum basilicum – Sweet, or Genovese basil; classic summer growing annual Ocimum ‘Pesto Perpetuo’ – variegated non-blooming basil! Ocimum ‘African Blue’ - sterile Rosmarinus officinalis ‘Blue Spires’ – upright grower, with large leaves, beautiful for standards Salvia officinalis ‘Berggarten’ – sun; classic culinary, with large gray leaves, very decorative Thymus vulgaris ‘English Wedgewood’ – sturdy culinary, easy to grow in ground or containers Artemesia dracunculus var sativa – French tarragon; herbaceous perennial. Absolutely needs great drainage! Origanum vulgare – Italian oregano, popular oregano flavor, evergreen; Greek oregano - strong flavor Mentha spicata ‘Kentucky Colonel’ – one of many, including ginger mint and orange mint Cymbopogon citratus – Lemon grass, great for cooking, and for dogs Aloysia triphylla – Lemon verbena ; Aloysia virgata – Sweet Almond Verbena – almond scented! Polygonum odoratum – Vietnamese coriander, a great perennial substitute for cilantro Agastache foeniculum ‘Blue Fortune’ – Anise hyssop, great for teas, honebee plant Agastache ‘Coronado’; A. Grape Nectar’ – both are 18 inches, delicious for tea, edible flr Agastache ‘Summer Breeze’ – large growing, full sun, bicolored pink and coral flowers Prostanthera rotundifolium – Australian Mint Bush. -

Effect of Different Extraction Methods on Yield and Quality of Essential Oil from Four Rosa Species
Floriculture and Ornamental Biotechnology ©2007 Global Science Books Effect of Different Extraction Methods on Yield and Quality of Essential Oil from Four Rosa Species Adnan Younis1* • Muhammad Aslam Khan1 • Asif Ali Khan2 • Atif Riaz1 • M. Aslam Pervez1 1 Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan 2 Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad, Pakistan Corresponding author : * [email protected] ABSTRACT In the present study rose oil was extracted from the petals of four Rosa species i.e. R. damascena, R. centifolia, R. borboniana and Rosa 'Gruss an Teplitz' through solvent extraction through hexane, solvent extraction through ether and steam distillation. R. damascena yielded (0.145%) of absolute oil, R. centifolia yielded 0.11% whereas R. 'Gruss an Teplitz' yielded the least (0.035%) absolute oil. Solvent extraction through hexane yielded more absolute oil (0.11%) than steam distillation (0.075%) and solvent extraction (0.07%) through ether on petal weight basis. Gas-chromatography of the rose oil was carried out for the qualitative and quantitative analysis of the oil constituents. Major compounds identified were citronellol, methyl eugenol, geraniol, geranyl acetate, phenyl ethyl alcohol, linalool, benzaldehyde, benzyl alcohol, rhodinyl acetate, citronellyl acetate, benzyl acetate and phenyl ethyl formate. Both techniques (solvent extraction and steam distillation) yielded oil with differences in the percentage composition of each component, but solvent extraction through hexane proved better (i.e. higher yield and more components) than steam distillation for extraction of essential oil from roses. _____________________________________________________________________________________________________________ Keywords: citronellol, essential oil composition, Rosa centifolia, solvent extraction, steam distillation INTRODUCTION essential oil, which is slowly liberated from the plant material (Durst and Gokel 1987; Wilson 1995). -

10 June 1954 CORRIGENDA to the CONSOLIDATED SCHEDULES 1. There Is Herewith Circulated to Each Contracting Party a Draft of the C
10 June 1954 CORRIGENDA TO THE CONSOLIDATED SCHEDULES 1. There is herewith circulated to each contracting party a draft of the corrections to be made to the Consolidated Schedules in order to keep them abreast of the legal texts annexed to the General Agreement. 2. It has not been thought necessary to prepare a new page for every change. This procedure would have entailed the risk of introducing further errors. In the case of the Schedule of the Belgian Congo and Ruanda Urundi (Schedule II, Section B), however, where the whole Section has been modified, a new Section has been prepared to replace the original. 3. Accordingly, there has been prepared for each consolidated schedule which requires amendment - as listed overleaf - (i) the items which have been modified or which require substantial amendment and the new items which are to be inserted, (the texts reproduced are to replace the whole of the corresponding items or sub-items in the Consolidated Schedules, unless otherwise indicated), and (ii) a list of alterations, such as changes in punctuation marks or item numbers, deletions of items, changes of single words, etc., which can be made more quickly and conveniently by hand. 4. For changes which arise out of rectifications or modifications contained in protocols, documents, etc., the source can be conveniently traced through the "List of Changes effected by Protocols and Decisions of the CONTRACTING PARTIES" (G/75). 5. Contracting parties are requested to examine the draft and to report any additions, corrections, etc., by 10 July 1954. By that date all contracting parties should indicate the number of photo-offset copies re^ulred-.- M3T/11/54 TABLE OF CONTENTS iÈSS. -

The Effect of Supplementing Diets with Dried Fennel and Thyme on the Zootechnical Parameters and Caecal Microflora of Growing Rabbit
Journal of Animal and Feed Sciences, 23, 2014, 346–350 The Kielanowski Institute of Animal Physiology and Nutrition, PAS, Jabłonna The effect of supplementing diets with dried fennel and thyme on the zootechnical parameters and caecal microflora of growing rabbit M. Benlemlih, A. Aarab, M. Bakkali, A. Arakrak and A. Laglaoui1 Abdelmalek Essaâdi University, Faculty of Science and Technology 416 Tangier, Morocco KEY WORDS: rabbit, fennel, thyme, growth ABSTRACT. The objective of this study was to evaluate the effect of fennel parameters, caecal microflora and thyme dietary supplements on the feeding of rabbits. Eighty-five weaned rabbits (35 days old), white New Zealand (of both sexes), were divided into four groups and submitted to the following dietary treatments: Control diet, diet F (Control diet + 2.5% Foeniculum vulgare seeds), diet T (Control diet + 2.5% Received: 2 December 2013 Thymus capitatus leaves) and diet FT (Control diet + 2.5% Foeniculum vulgare Revised: 13 November 2014 seeds and Thymus capitatus leaves) for twenty-five days. The treatment with Accepted: 28 November 2014 fennel and thyme had a beneficial effect on the mortality rate (18%). However the growth rate, feed conversion ratio and carcass yield were not influenced by dietary fennel and/or thyme supplementation. The antimicrobial effect of thyme (2.5%) was observed only against C. perfringens in the caecum (P < 0.05), but 1 Corresponding author: no effect was observed on the caecal count of or C. perfringens in the other e-mail: [email protected] treated groups. Introduction to their different active substances. The influence of some medicinal plants and their extracts on rabbit Weaning is the most critical period in rabbit has been studied (Eiben et al., 2004; El-Nattat and breeding; it is associated with a higher risk of El-Kady, 2007; Soultos et al., 2009; Simonová digestive disorders in growing rabbits (Krieg et al., et al., 2010; Gerencsér et al., 2012). -

21 CFR Ch. I (4–1–10 Edition) § 582.20
§ 582.20 21 CFR Ch. I (4–1–10 Edition) Common name Botanical name of plant source Marjoram, sweet .......................................................................... Majorana hortensis Moench. Mustard, black or brown .............................................................. Brassica nigra (L.) Koch. Mustard, brown ............................................................................ Brassica juncea (L.) Coss. Mustard, white or yellow .............................................................. Brassica hirta Moench. Nutmeg ........................................................................................ Myristica fragrans Houtt. Oregano (oreganum, Mexican oregano, Mexican sage, origan) Lippia spp. Paprika ......................................................................................... Capsicum annuum L. Parsley ......................................................................................... Petroselinum crispum (Mill.) Mansf. Pepper, black ............................................................................... Piper nigrum L. Pepper, cayenne ......................................................................... Capsicum frutescens L. or Capsicum annuum L. Pepper, red .................................................................................. Do. Pepper, white ............................................................................... Piper nigrum L. Peppermint .................................................................................. Mentha piperita L. Poppy seed -
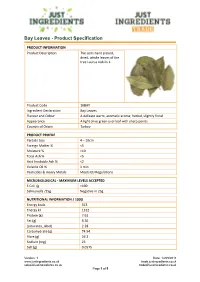
Bay Leaves - Product Specification
Bay Leaves - Product Specification PRODUCT INFORMATION Product Description The semi hand picked, dried, whole leaves of the tree Laurus nobilis L. Product Code 10BAY Ingredient Declaration Bay Leaves Flavour and Odour A delicate warm, aromatic aroma; herbal, slightly floral Appearance A light olive green oval leaf with sharp points Country of Origin Turkey PRODUCT PROFILE Particle Size 4 – 10cm Foreign Matter % <5 Moisture % <10 Total Ash % <5 Acid Insoluble Ash % <2 Volatile Oil % 1 min Pesticides & Heavy Metals Meets EU Regulations MICROBIOLOGICAL - MAXIMUM LEVELS ACCEPTED E Coli /g <100 Salmonella /25g Negative in 25g NUTRITIONAL INFORMATION / 100G Energy kcals 313 Energy kJ 1312 Protein (g) 7.61 Fat (g) 8.36 {saturates_label} 2.28 Carbohydrate (g) 74.94 Fibre (g) 26.3 Sodium (mg) 23 Salt (g) 0.0575 Version: 1 Date: 14/05/2018 www.justingredients.co.uk trade.justingredients.co.uk [email protected] [email protected] Page 1 of 5 Bay Leaves - Product Specification INTOLERANCE AND ALLERGEN INFORMATION Please ensure you have read our Allergen Policy statement available here. For further information about allergen handling by JustIngredients and its suppliers, please read our online guide here. Key: ✓ Indicates where a product is an allergen or where an allergen has intentionally been added to the final product. Cereal/Wheat products Nut and nut products Peanuts and products thereof Soybean and products thereof Sesame seed and products thereof Mustard and products thereof Milk and Dairy Products Products containing Sulphur -

Savory Guide
The Herb Society of America's Essential Guide to Savory 2015 Herb of the Year 1 Introduction As with previous publications of The Herb Society of America's Essential Guides we have developed The Herb Society of America's Essential The Herb Society Guide to Savory in order to promote the knowledge, of America is use, and delight of herbs - the Society's mission. We hope that this guide will be a starting point for studies dedicated to the of savory and that you will develop an understanding and appreciation of what we, the editors, deem to be an knowledge, use underutilized herb in our modern times. and delight of In starting to put this guide together we first had to ask ourselves what it would cover. Unlike dill, herbs through horseradish, or rosemary, savory is not one distinct species. It is a general term that covers mainly the educational genus Satureja, but as time and botanists have fractured the many plants that have been called programs, savories, the title now refers to multiple genera. As research and some of the most important savories still belong to the genus Satureja our main focus will be on those plants, sharing the but we will also include some of their close cousins. The more the merrier! experience of its Savories are very historical plants and have long been utilized in their native regions of southern members with the Europe, western Asia, and parts of North America. It community. is our hope that all members of The Herb Society of America who don't already grow and use savories will grow at least one of them in the year 2015 and try cooking with it. -

Essential Oil Composition of Two Greek Cultivated Sideritis Spp
Nat. Volatiles & Essent. Oils, 2019; 6(3): 16-23 Kloukina et al. RESEARCH ARTICLE Essential oil composition of two Greek cultivated Sideritis spp. Charalampia Kloukina, Ekaterina-Michaela Tomou, Helen Skaltsa* Department of Pharmacognosy & Chemistry of Natural Products, School of Pharmacy, National and Kapodistrian University of Athens, Athens, Greece *Corresponding author. Email: [email protected] Abstract In this present work, essential oil composition of cultivated commercially available Sideritis raeseri Boiss. & Heldr. and Sideritis scardica Griseb. from three different restricted areas of Kozani (central Greece) were studied. The essential oils were obtained by hydrodistillation in a modified Clevenger-type apparatus, and their analyses were performed by GC-MS. Monoterpene hydrocarbons constituted the main fraction of S. raeseri from Polirraxo (Kozani) and of S. scardica from Chromio (Kozani), while sesquiterpene hydrocarbons were the main group of S. raeseri from Metamorfosis (Kozani) and S. scardica from Metamorfosis (Kozani). Despite the different cultivations, some constituents were found even in different percentages in both samples of S. scardica: α-pinene, -pinene, cis-caryophyllene, bicyclogermacrene and germacrene D. It is noteworthy that the two samples of S. raeseri have totally different main constituents, which could be related to the different cultivation conditions, as well as to the known tendency of some Sideritis species to hybridize, which suggests further research. Keywords: Sideritis raeseri, Sideritis scardica, cultivation, composition of volatiles Introduction Sideritis L. (Lamiaceae) comprises more than 150 species, indigenous in Central Europe, the Mediterranean, the Balkans, the Iberian Peninsula and the west Asia (González-Burgos et al., 2011). Traditionally, the infusion of herba Sideritis (S. scardica, S. -

Assessment Report on Salvia Officinalis L., Folium and Salvia Officinalis L., Aetheroleum Final
20 September 2016 EMA/HMPC/150801/2015 Committee on Herbal Medicinal Products (HMPC) Assessment report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum Final Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC (traditional use) Herbal substance(s) (binomial scientific name of Salvia officinalis L., folium and the plant, including plant part) Salvia officinalis L., aetheroleum Herbal preparation(s) a) Comminuted herbal substance b) Liquid extract (DER 1:1), extraction solvent ethanol 70% V/V c) Dry extract (DER 4-7:1), extraction solvent water d) Liquid extract (DER 1:3.5-5), extraction solvent ethanol 31.5% V/V e) Liquid extract (DER 1:4-5) extraction solvent ethanol 50% V/V f) Liquid extract (DER 1:4-6), extraction solvent liquor wine:ethanol 96% V/V (38.25:61.75 m/m) g) Tincture (ratio of herbal substance to extraction solvent 1:10) extraction solvent ethanol 70% V/V Pharmaceutical form(s) Comminuted herbal substance as herbal tea for oral use. Comminuted herbal substance for infusion preparation for oromucosal or cutaneous use. Herbal preparations in solid or liquid dosage forms for oral use. Herbal preparations in liquid or semi-solid dosage forms for cutaneous use or for oromucosal use. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2017. Reproduction is authorised provided the source is acknowledged. -

Sideritis Clandestina (Bory & Chaub.) Hayek; Sideritis Raeseri Boiss
2 February 2016 EMA/HMPC/39455/2015 Committee on Herbal Medicinal Products (HMPC) Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba Final Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use) Herbal substances (binomial scientific name of Sideritis scardica Griseb.; Sideritis clandestina the plant, including plant part) (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba Herbal preparation Comminuted herbal substance Pharmaceutical form Comminuted herbal substance as herbal tea for oral use Rapporteur I. Chinou Peer-reviewer B. Kroes 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ................................................................................................................... 2 1. Introduction ....................................................................................................................... 4 1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof .. 4 1.2. Search and assessment methodology ..................................................................... 8 2. Data on