21 CFR Ch. I (4–1–10 Edition) § 582.20
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tamarind 1990 - 2004
Tamarind 1990 - 2004 Author A. K. A. Dandjouma, C. Tchiegang, C. Kapseu and R. Ndjouenkeu Title Ricinodendron heudelotii (Bail.) Pierre ex Pax seeds treatments influence on the q Year 2004 Source title Rivista Italiana delle Sostanze Grasse Reference 81(5): 299-303 Abstract The effects of heating Ricinodendron heudelotii seeds on the quality of the oil extracted was studied. The seeds were preheated by dry and wet methods at three temperatures (50, 70 and 90 degrees C) for 10, 20, 30 and 60 minutes. The oil was extracted using the Soxhlet method with hexane. The results showed a significant change in oil acid value when heated at 90 degrees C for 60 minutes, with values of 2.76+or-0.18 for the dry method and 2.90+or-0.14 for the wet method. Heating at the same conditions yielded peroxide values of 10.70+or-0.03 for the dry method and 11.95+or-0.08 for the wet method. Author A. L. Khandare, U. Kumar P, R. G. Shanker, K. Venkaiah and N. Lakshmaiah Title Additional beneficial effect of tamarind ingestion over defluoridated water supply Year 2004 Source title Nutrition Reference 20(5): 433-436 Abstract Objective: We evaluated the effect of tamarind (Tamarindus indicus) on ingestion and whether it provides additional beneficial effects on mobilization of fluoride from the bone after children are provided defluoridated water. Methods: A randomized, diet control study was conducted in 30 subjects from a fluoride endemic area after significantly decreasing urinary fluoride excretion by supplying defluoridated water for 2 wk. -

Comparative Mapping Between Arabidopsis Thaliana and Brassica Nigra Indicates That Brassica Genomes Have Evolved Through Extensi
Copyright 1998 by the Genetics Society of America Comparative Mapping Between Arabidopsis thaliana and Brassica nigra Indicates That Brassica Genomes Have Evolved Through Extensive Genome Replication Accompanied by Chromosome Fusions and Frequent Rearrangements Ulf Lagercrantz Department of Plant Biology, Swedish University of Agricultural Sciences, S-750 07 Uppsala, Sweden Manuscript received March 27, 1998 Accepted for publication July 24, 1998 ABSTRACT Chromosome organization and evolution in the Brassicaceae family was studied using comparative linkage mapping. A total of 160 mapped Arabidopsis thaliana DNA fragments identi®ed 284 homologous loci covering 751 cM in Brassica nigra. The data support that modern diploid Brassica species are descended from a hexaploid ancestor, and that the A. thaliana genome is similar in structure and complexity to those of each of the hypothetical diploid progenitors of the proposed hexaploid. Thus, the Brassica lineage probably went through a triplication after the divergence of the lineages leading to A. thaliana and B. nigra. These duplications were also accompanied by an exceptionally high rate of chromosomal rearrangements. The average length of conserved segments between A. thaliana and B. nigra was estimated at 8 cM. This estimate corresponds to z90 rearrangements since the divergence of the two species. The estimated rate of chromosomal rearrangements is higher than any previously reported data based on comparative mapping. Despite the large number of rearrangements, ®ne-scale comparative mapping between model plant A. thal- iana and Brassica crops is likely to result in the identi®cation of a large number of genes that affect important traits in Brassica crops. NE important aspect of genome evolution is polyploid (Masterson 1994). -

Effect of Feeding Brassica Juncea Seeds on Experimentally Induced Hyperlipidemia
EMAD-UD-DIN ET AL (2014), FUUAST J. BIOL., 4(1): 61-64 EFFECT OF FEEDING BRASSICA JUNCEA SEEDS ON EXPERIMENTALLY INDUCED HYPERLIPIDEMIA MUHAMMAD EMAD-UD-DIN, GHAZALA YASMEEN, NAZISH IQBAL KHAN AND LUBNA NAZ Pathophysiology Unit, Department of Physiology, University of Karachi, Karachi, Pakistan. Corresponding Authors e-mail: [email protected] Abstract Cardiovascular Disease (CVD) is major cause of death worldwide. Atherosclerosis is principal underlying mechanism, stimulated by hyperlipidemia. Substantial evidences suggest that atherosclerosis can be prevented or at least retarded by controlling serum lipids, especially cholesterol, at initial stages. The study was aimed to investigate the antihyperlipidemic potential of Brassica juncea seeds in cholesterol-fed rabbits owing to expected lipid lowering effect of B. juncea seeds. Age matched 18 rabbits were randomly divided into three equal groups control, hyperlipidemic and treated. Following one week acclimatization, control group kept on normal animal chow, 5% atherogenic diet was administered to hyperlipidemic group while treated were fed 10% dried Brassica juncea seeds along with atherogenic diet for eight weeks. Blood specimens were obtained and assayed for lipid profile (TC, HDL-C, LDL-C, TG, vLDL, TC/HDL-C). Data was analyzed using t-test at 0.05. High cholesterol diet significantly increased plasma TC (p<0.01, 60.25%), LDL-C (p<0.05, 10.8%), TG (p<0.05, 19.9%) while rise in HDL-C (23.1%) and vLDL (18.4%) remained non-significant as compared with control. Brassica juncea seeds consumption showed significant lipid lowering activity TC (p<0.05, -24.7%), HDL-C (p<0.05, 36.7%), LDL-C (p<0.05, -34%), TG (p<0.01, -41%) and vLDL (p<0.05, -40.7%) in comparison to hyperlipidemic counterparts. -

Brassicaceae Biofumigation for Weeds and Soil-Borne Diseases In
Brassicaceae Biofumigation for Weeds and Soil-Borne Diseases in Chile Pepper: On-Farm Evaluations of a Mustard Cover Crop Asmita Nagila, MS student in Agricultural Biology; Brian Schutte & Soum Sanogo, NMSU Dept. Entomology, Plant Pathology and Weed Science John Idowu, NMSU Dept. Extension Plant Sciences STUDY SITES COVER CROP BIOMASS AT MUSTARD TERMINATION Cover Crop Mustard Cover Crop Average First Cover Crop Study Site Termination Site-Specific Alternative Frost Date Seeding Date 600 Date Figure 1. Aboveground biomass for mustard cover crop and site-specific alternative cover crops grown at Columbus, NM Nov. 1 – Nov. 10 Nov. 10, 2018 Feb. 14, 2019 commercial farms (Columbus, 400 Deming and Las Uvas) and a Deming, NM Oct. 21 – Oct. 31 Sept. 29, 2018 Feb. 22, 2019 -2 university research farm (Leyendecker) in southern New g m g Las Uvas, NM Oct. 21 – Oct. 31 Oct. 29, 2018 March 5, 2019 Mexico. Bars are means with 200 NMSU standard errors (N = 18). Nov. 1 – Nov. 10 Oct. 11, 2018 March 15, 2019 Leyendecker Aboveground biomass 0 TREATMENTS Columbus Deming Las Uvas Leyendecker • Mustard Cover Crop • Site-Specific Alternative • No Cover Crop Columbus Las Uvas Mustard Cover Crop: Mixture of caliente ‘rojo’ (Brassica juncea cv ‘rojo’) and arugula (Eruca sativa) Site-Specific Alternatives: o Columbus: Barley o Deming: Mustard with wheat Barley Mustard Barley Mustard o Las Uvas: Barley COVER CROP MEASUREMENTS CARRYOVER EFFECTS ON WEED SEEDBANK • Biomass at mustard termination • Weed biomass at mustard termination Mustard Cover Crop 100 No Cover Crop 60 • Glucosinolate content (pesticidal component of A B mustard cover crop) 80 40 CARRYOVER EFFECTS ON WEED SEEDBANKS 60 • Palmer amaranth seed persistence in buried packets 40 • Germination of persistent Palmer amaranth seeds 20 (laboratory) Germination Seed persistence 20 (% of seeds (% of buried) (% of persistent(% seeds) 0 0 Columbus Deming Leyendecker Columbus Deming Leyendecker Figure 2. -

Building Big Flavor
BUILDING BIG FLAVOR Watching your sodium intake? Balancing flavors is the key to a flavorful meal when reducing the salt in a dish. Think about adding these flavor enhancers instead of reaching for the salt shaker! Sweet Bitter Acidic Umami (Savory) Brings balance and Balances sweetness Brings brightness Makes a dish savory or roundness to a dish by and cuts richness - and adds a salty meaty tasting and balancing acidity and best used as flavor that enhances flavors - reach bitterness and background flavor balances for these before salt! highlighting other flavors sweetness Fruit juices, Nectars, Greens (Kale, Lemon, Lime, Tomato Products Concentrates, Chard, Dandelion, Orange and (especially canned, like Reductions, Caramelized Chicory, Watercress, Pineapple Juice, paste) Soy Sauce, Onions, Carrots, Sweet Arugula) Broccoli Vinegars, Wine, Mushrooms (especially Potatoes, Butternut Rabe, Broccoli, Tamarind, dried) Cured or brined Squash, Roasted Cabbage, Brussels Pickled Foods, foods (olives) Seaweed, Peppers, Honey, Maple Sprouts, Asparagus, Cranberries, Sour Fish Sauce, Fermented Syrup, Molasses, Dried Some Mustards, Cherries, Tomato Foods (Miso, Fermented Fruits, Tomato Paste, Grapefruit, Citrus Products Black beans, Sauerkraut) Beets, Reduced Vinegars, Rind/Zest, Beer, Aged cheeses (Parmesan, Wine, Wine, Teas (black & Blue, Gouda) Liquid Amino green) Acids, Seafood (especially dried), Worcestershire Sauce, Anchovy, Beef, Pork (especially cured), Chicken Don’t forget to read nutrition labels and watch for foods that are commonly high -

Anti–Oxidative and Anti–Inflammatory Effects of Tagetes Minuta Essential Oil in Activated Macrophages
Asian Pac J Trop Biomed 2014; 4(3): 219-227 219 Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Biomedicine journal homepage: www.elsevier.com/locate/apjtb Document heading doi:10.1016/S2221-1691(14)60235-5 2014 by the Asian Pacific Journal of Tropical Biomedicine. All rights reserved. 襃 Anti-oxidative and anti-inflammatory effects of Tagetes minuta essential oil in activated macrophages 1 1 2 Parastoo Karimian , Gholamreza Kavoosi *, Zahra Amirghofran 1Biotechnology Institute, Shiraz University, Shiraz, 71441-65186, Iran 2Department of Immunology, Autoimmune Disease Research Center and Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, Shiraz, Iran PEER REVIEW ABSTRACT Peer reviewer Objective: Tagetes minuta T. minuta To investigate antioxidant and anti-inflammatory effects of ( ) Hasan Salehi, University of Shiraz, essentialMethods: oil. T. minuta T. minuta Shiraz, Iran. In the present study essential oil was obtained from leaves of via E-mail: [email protected] hydro-distillation andT. minutathen was analyzed by gas chromatography-mass spectrometry. The anti- Comments oxidant capacity of essential oil was examined by measuring reactive oxygen,T. reactive minuta nitrogen species and hydrogen peroxide scavenging. The anti-inflammatory activity of TMO displayed an anti-oxidant essential αoil was determined through measuring NADH oxidase, inducible nitric oxide synthase property by scavenging superoxide, and TNF- mRNA expression in lipopolysacharide-stimulated murine macrophages using real- H2O2 and NO radicals, and reduced Results:time PCR. G oxidative stress. The decreased T. minutaas chromatography-mass spectrometry analysis indicated that the main components in ROS NOS (33 86%) E (19 92%) (16 15%) formation of and radicals in the β essential oil were dihydrotagetone . -

Butter Poached Prawns with Tarragon & Garlic
Butter Poached Prawns with Tarragon & Garlic the One° Precision Poacher™ With probe Butter Poached Shrimp with Tarragon & Garlic Prep 5 minutes / Cook 15 minutes the One° Precision Poacher™ Serves 2 16 large shrimp, peeled and deveined, tails intact 4 tablespoons (60g) salted butter, diced 1 tablespoon fresh tarragon, finely chopped 1 clove garlic, crushed Freshly ground black pepper, to taste Method 1. Fill the pot of the Precision Poacher with water up to the SOUS VIDE fill line. Put the egg tray into the pot. Cover with the lid and insert probe through the vent. Press METHOD button to select SOUS VIDE. Press TEMPERATURE button to select 59°C. Press TIME button to select 15 minutes. Press START to preheat water. 2. While the water is preheating, place shrimp neatly into a vacuum bag with butter cubes, tarragon, garlic and black pepper. Vacuum seal the bag. 3. When preheat has finished, the unit will beep. Drop the bag into the water, ensuring it is submerged. Cover with the lid and insert probe. 4. Press START. When cooking is complete, snip the bag and divide shrimps among two bowls. Drizzle over the garlic and tarragon butter, season. Serve with crusty bread and salad. Note: A vacuum sealer and vacuum bags are needed for this recipe. BEG800 B16 Eggs Benedict the One° Precision Poacher™ With probe Eggs Benedict Prep 10 minutes / Cook 20 minutes Serves 4 (Makes ¾ cup (200ml) hollandaise) the One° Precision Poacher™ 4 large eggs 1 tablespoon olive oil 4 portobello mushrooms 4oz (115g) shaved smoked ham 1 bunch (200g) spinach, washed and trimmed Hollandaise 3 large egg yolks 2 tablespoons lemon juice 7 tablespoons (100g) unsalted butter, cubed Salt and pepper, to season Method 1. -

Tolerance of Vegetable Crops to Salinity M.C
Scientia Horticulturae 78 (1999) 5±38 Tolerance of vegetable crops to salinity M.C. Shannon*, C.M. Grieve U.S. Salinity Laboratory, Department of Agriculture, Agricultural Research Service, 450 W. Big Springs Road, Riverside, CA 92507, USA Abstract Global constraints on fresh water supplies and the need to dispose of agricultural, municipal, and industrial waste waters have intensified interest in water reuse options. In many instances, the value of the water is decreased solely because of its higher salt concentration. Although quantitative information on crop salt tolerance exists for over 130 crop species, there are many vegetables which lack definitive data. Vegetable crops are defined as herbaceous species grown for human consumption in which the edible portions consist of leaves, roots, hypocotyls, stems, petioles, and flower buds. The salt tolerance of vegetable species is important because the cash value of vegetables is usually high compared to field crops. In this review some general information is presented on how salinity affects plant growth and development and how different measurements of salinity in solution cultures, sand cultures, and field studies can be reconciled to a common basis. The salt tolerance of vegetables has been condensed and reported in a uniform format based on the best available data. Discrepancies and inconsistencies exist in some of the information due to differences in cultivars, environments, and experimental conditions. For a great number of species little or no useful information exists and there is an obvious need for research. Published by Elsevier Science B.V. Keywords: Salt tolerance; Ion composition Contents 1. Introduction ............................................................ 7 1.1. -

Identification of a Scar Marker Linked to a Shattering Resistance Trait in Sesame
Turk J Field Crops 2017, 22(2), 258-265 DOI: 10.17557/tjfc.359707 IDENTIFICATION OF A SCAR MARKER LINKED TO A SHATTERING RESISTANCE TRAIT IN SESAME Chalermpol PHUMICHAI1*, Weerachai MATTHAYATTHAWORN1, Nipha CHUENPOM1, Arunee WONGKAEW1, Phakaked SOMSAENG1, Tanapong YODYINGYONG1, Pherawich, PANKLANG1, Sujin JENWEERAWAT1, Yaowamarn KEAWSAARD1, Thitaporn PHUMICHAI2, Tanee SREEWONGCHAI1, Rangsarid KAVEETA1 1Kasetsart University, Faculty of Agriculture, Department of Agronomy, Bangkok, THAILAND. 2Rubber Research Institute of Thailand, Bangkok, THAILAND. *Corresponding author: [email protected] Received: 09.08.2017 ABSTRACT Sesame (Sesamum indicum L.) is one of the most important oil crops in temperate and tropical regions and is grown worldwide over an area of 5179 (hg ha-1) to produce 5.469.024 tonnes of seed. Capsule shattering before or during harvest can cause yield losses of greater than 50%. The objectives of the present study were to evaluate the inheritance of resistance to capsule shattering in the F2 derived from a cross between Cplus1, a sesame line with shattering-resistant capsules (Sh1Sh1Sh2Sh2), and KUAOX25, a line with shattering- susceptible capsules (sh1sh1sh2sh2-); and use bulked segregant analysis and Amplified Fragment Length Polymorphisms (AFLPs) to identify Sequence Characterized Amplified Region (SCAR) markers associated with shattering resistance. After screening 192 AFLP primer combinations, nine polymorphic bands were identified, and one of these AFLPs was developed into a Si-SR-32-19 SCAR that could distinguish between shattering-susceptible and shattering-resistant phenotypes. Keywords: AFLP, BSA, SCAR, Sesamum indicum L., shattering resistance INTRODUCTION production of sesame is estimated at 5.469.024 tonnes, and the average annual yield worldwide is roughly 5179 Sesame (Sesamum indicum L.), a member of the (hg ha-1) (FAOSTAT, 2014) Pedaliaceae family, is an important oilseed crop that is widely cultivated in tropical and subtropical areas (Ashri, The production of sesame is limited due to capsule 2010). -

10 June 1954 CORRIGENDA to the CONSOLIDATED SCHEDULES 1. There Is Herewith Circulated to Each Contracting Party a Draft of the C
10 June 1954 CORRIGENDA TO THE CONSOLIDATED SCHEDULES 1. There is herewith circulated to each contracting party a draft of the corrections to be made to the Consolidated Schedules in order to keep them abreast of the legal texts annexed to the General Agreement. 2. It has not been thought necessary to prepare a new page for every change. This procedure would have entailed the risk of introducing further errors. In the case of the Schedule of the Belgian Congo and Ruanda Urundi (Schedule II, Section B), however, where the whole Section has been modified, a new Section has been prepared to replace the original. 3. Accordingly, there has been prepared for each consolidated schedule which requires amendment - as listed overleaf - (i) the items which have been modified or which require substantial amendment and the new items which are to be inserted, (the texts reproduced are to replace the whole of the corresponding items or sub-items in the Consolidated Schedules, unless otherwise indicated), and (ii) a list of alterations, such as changes in punctuation marks or item numbers, deletions of items, changes of single words, etc., which can be made more quickly and conveniently by hand. 4. For changes which arise out of rectifications or modifications contained in protocols, documents, etc., the source can be conveniently traced through the "List of Changes effected by Protocols and Decisions of the CONTRACTING PARTIES" (G/75). 5. Contracting parties are requested to examine the draft and to report any additions, corrections, etc., by 10 July 1954. By that date all contracting parties should indicate the number of photo-offset copies re^ulred-.- M3T/11/54 TABLE OF CONTENTS iÈSS. -
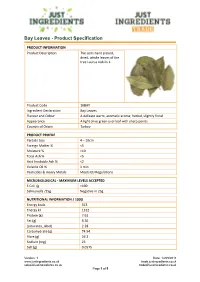
Bay Leaves - Product Specification
Bay Leaves - Product Specification PRODUCT INFORMATION Product Description The semi hand picked, dried, whole leaves of the tree Laurus nobilis L. Product Code 10BAY Ingredient Declaration Bay Leaves Flavour and Odour A delicate warm, aromatic aroma; herbal, slightly floral Appearance A light olive green oval leaf with sharp points Country of Origin Turkey PRODUCT PROFILE Particle Size 4 – 10cm Foreign Matter % <5 Moisture % <10 Total Ash % <5 Acid Insoluble Ash % <2 Volatile Oil % 1 min Pesticides & Heavy Metals Meets EU Regulations MICROBIOLOGICAL - MAXIMUM LEVELS ACCEPTED E Coli /g <100 Salmonella /25g Negative in 25g NUTRITIONAL INFORMATION / 100G Energy kcals 313 Energy kJ 1312 Protein (g) 7.61 Fat (g) 8.36 {saturates_label} 2.28 Carbohydrate (g) 74.94 Fibre (g) 26.3 Sodium (mg) 23 Salt (g) 0.0575 Version: 1 Date: 14/05/2018 www.justingredients.co.uk trade.justingredients.co.uk [email protected] [email protected] Page 1 of 5 Bay Leaves - Product Specification INTOLERANCE AND ALLERGEN INFORMATION Please ensure you have read our Allergen Policy statement available here. For further information about allergen handling by JustIngredients and its suppliers, please read our online guide here. Key: ✓ Indicates where a product is an allergen or where an allergen has intentionally been added to the final product. Cereal/Wheat products Nut and nut products Peanuts and products thereof Soybean and products thereof Sesame seed and products thereof Mustard and products thereof Milk and Dairy Products Products containing Sulphur -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa