Coriander Fruit. I Yield and Glucosinolate Contents of Mustard (Sinapis Sp., Brassica Sp.) Seeds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Mapping Between Arabidopsis Thaliana and Brassica Nigra Indicates That Brassica Genomes Have Evolved Through Extensi
Copyright 1998 by the Genetics Society of America Comparative Mapping Between Arabidopsis thaliana and Brassica nigra Indicates That Brassica Genomes Have Evolved Through Extensive Genome Replication Accompanied by Chromosome Fusions and Frequent Rearrangements Ulf Lagercrantz Department of Plant Biology, Swedish University of Agricultural Sciences, S-750 07 Uppsala, Sweden Manuscript received March 27, 1998 Accepted for publication July 24, 1998 ABSTRACT Chromosome organization and evolution in the Brassicaceae family was studied using comparative linkage mapping. A total of 160 mapped Arabidopsis thaliana DNA fragments identi®ed 284 homologous loci covering 751 cM in Brassica nigra. The data support that modern diploid Brassica species are descended from a hexaploid ancestor, and that the A. thaliana genome is similar in structure and complexity to those of each of the hypothetical diploid progenitors of the proposed hexaploid. Thus, the Brassica lineage probably went through a triplication after the divergence of the lineages leading to A. thaliana and B. nigra. These duplications were also accompanied by an exceptionally high rate of chromosomal rearrangements. The average length of conserved segments between A. thaliana and B. nigra was estimated at 8 cM. This estimate corresponds to z90 rearrangements since the divergence of the two species. The estimated rate of chromosomal rearrangements is higher than any previously reported data based on comparative mapping. Despite the large number of rearrangements, ®ne-scale comparative mapping between model plant A. thal- iana and Brassica crops is likely to result in the identi®cation of a large number of genes that affect important traits in Brassica crops. NE important aspect of genome evolution is polyploid (Masterson 1994). -

Tips for Cooking with Coriander / Cilantro Russian Green Bean Salad
Recipes Tips for Cooking with Coriander / Cilantro • Gently heat seeds in a dry pan until fragrant before crushing or grinding to enhance the flavor. • Crush seeds using a mortar and pestle or grind seeds in a spice mill or coffee grinder. • Seeds are used whole in pickling recipes. • Cilantro is best used fresh as it loses flavor when dried. • Clean cilantro bunches by swishing the leaves in water and patting dry. • For the best color, flavor and texture, add cilantro leaves towards the end of the cooking time. • The stems have flavor too, so tender stems may be chopped and added along with the leaves. • Store cilantro stem in a glass of water in the refrigerator, with a loose plastic bag over the top. Russian Green Bean Salad with Garlic, Walnuts, Basil, Cilantro and Coriander Seed ½ cup broken walnuts ¼ cup firmly packed basil leaves 2 large cloves garlic, peeled and each cut into ¼ cup firmly packed cilantro leaves and several pieces tender stems 4 Tbsp extra-virgin olive oil 1 pound fresh green beans, stems removed 2 Tbsp white wine vinegar and steamed until crisp – tender and cooled 1 Tbsp lemon juice in ice water 1 Tbsp water ½ cup thinly sliced green onions 1 tsp ground coriander seed ½ cup thinly sliced radishes ⅛ to ¼ tsp hot pepper sauce such as Tabasco Salt and freshly ground pepper to taste 2 Tbsp firmly packed parsley leaves and tender stems To prepare dressing, place walnuts and garlic in food processor fitted with knife blade; chop, using pulse control, until evenly fine. Add olive oil, vinegar, lemon juice, water, coriander seed and hot pepper sauce; process until smooth. -

Effect of Feeding Brassica Juncea Seeds on Experimentally Induced Hyperlipidemia
EMAD-UD-DIN ET AL (2014), FUUAST J. BIOL., 4(1): 61-64 EFFECT OF FEEDING BRASSICA JUNCEA SEEDS ON EXPERIMENTALLY INDUCED HYPERLIPIDEMIA MUHAMMAD EMAD-UD-DIN, GHAZALA YASMEEN, NAZISH IQBAL KHAN AND LUBNA NAZ Pathophysiology Unit, Department of Physiology, University of Karachi, Karachi, Pakistan. Corresponding Authors e-mail: [email protected] Abstract Cardiovascular Disease (CVD) is major cause of death worldwide. Atherosclerosis is principal underlying mechanism, stimulated by hyperlipidemia. Substantial evidences suggest that atherosclerosis can be prevented or at least retarded by controlling serum lipids, especially cholesterol, at initial stages. The study was aimed to investigate the antihyperlipidemic potential of Brassica juncea seeds in cholesterol-fed rabbits owing to expected lipid lowering effect of B. juncea seeds. Age matched 18 rabbits were randomly divided into three equal groups control, hyperlipidemic and treated. Following one week acclimatization, control group kept on normal animal chow, 5% atherogenic diet was administered to hyperlipidemic group while treated were fed 10% dried Brassica juncea seeds along with atherogenic diet for eight weeks. Blood specimens were obtained and assayed for lipid profile (TC, HDL-C, LDL-C, TG, vLDL, TC/HDL-C). Data was analyzed using t-test at 0.05. High cholesterol diet significantly increased plasma TC (p<0.01, 60.25%), LDL-C (p<0.05, 10.8%), TG (p<0.05, 19.9%) while rise in HDL-C (23.1%) and vLDL (18.4%) remained non-significant as compared with control. Brassica juncea seeds consumption showed significant lipid lowering activity TC (p<0.05, -24.7%), HDL-C (p<0.05, 36.7%), LDL-C (p<0.05, -34%), TG (p<0.01, -41%) and vLDL (p<0.05, -40.7%) in comparison to hyperlipidemic counterparts. -

The Dispersal and Acclimatization of the Muskrat, Ondatra Zibethicus (L.), in Finland
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Wildlife Damage Management, Internet Center Other Publications in Wildlife Management for 1960 The dispersal and acclimatization of the muskrat, Ondatra zibethicus (L.), in Finland Atso Artimo Suomen Riistanhoito-Saatio (Finnish Game Foundation) Follow this and additional works at: https://digitalcommons.unl.edu/icwdmother Part of the Environmental Sciences Commons Artimo, Atso, "The dispersal and acclimatization of the muskrat, Ondatra zibethicus (L.), in Finland" (1960). Other Publications in Wildlife Management. 65. https://digitalcommons.unl.edu/icwdmother/65 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Other Publications in Wildlife Management by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. R I 1ST A TIE T L .~1 U ( K A I S U J A ,>""'liSt I " e'e 'I >~ ~··21' \. • ; I .. '. .' . .,~., . <)/ ." , ., Thedi$perscdQnd.a~C:li"'dti~otlin. of ,the , , :n~skret, Ond~trq ~ib.t~i~',{(.h in. Firtland , 8y: ATSO ARTIMO . RllSTATIETEELLISljX JULKAISUJA PAPERS ON GAME RESEARCH 21 The dispersal and acclimatization of the muskrat, Ondatra zibethicus (l.), in Finland By ATSO ARTIMO Helsinki 1960 SUOMEN FIN LANDS R I 1ST A N HOI T O-S A A T I b ] AK TV ARDSSTI FTELSE Riistantutkimuslaitos Viltforskningsinstitutet Helsinki, Unionink. 45 B Helsingfors, Unionsg. 45 B FINNISH GAME FOUNDATION Game Research Institute Helsinki, Unionink. 45 B Helsinki 1960 . K. F. Puromichen Kirjapaino O.-Y. The dispersal and acclimatization of the muskrat, Ondatra zibethicus (L.), in Finland By Atso Artimo CONTENTS I. -

Brassicaceae Biofumigation for Weeds and Soil-Borne Diseases In
Brassicaceae Biofumigation for Weeds and Soil-Borne Diseases in Chile Pepper: On-Farm Evaluations of a Mustard Cover Crop Asmita Nagila, MS student in Agricultural Biology; Brian Schutte & Soum Sanogo, NMSU Dept. Entomology, Plant Pathology and Weed Science John Idowu, NMSU Dept. Extension Plant Sciences STUDY SITES COVER CROP BIOMASS AT MUSTARD TERMINATION Cover Crop Mustard Cover Crop Average First Cover Crop Study Site Termination Site-Specific Alternative Frost Date Seeding Date 600 Date Figure 1. Aboveground biomass for mustard cover crop and site-specific alternative cover crops grown at Columbus, NM Nov. 1 – Nov. 10 Nov. 10, 2018 Feb. 14, 2019 commercial farms (Columbus, 400 Deming and Las Uvas) and a Deming, NM Oct. 21 – Oct. 31 Sept. 29, 2018 Feb. 22, 2019 -2 university research farm (Leyendecker) in southern New g m g Las Uvas, NM Oct. 21 – Oct. 31 Oct. 29, 2018 March 5, 2019 Mexico. Bars are means with 200 NMSU standard errors (N = 18). Nov. 1 – Nov. 10 Oct. 11, 2018 March 15, 2019 Leyendecker Aboveground biomass 0 TREATMENTS Columbus Deming Las Uvas Leyendecker • Mustard Cover Crop • Site-Specific Alternative • No Cover Crop Columbus Las Uvas Mustard Cover Crop: Mixture of caliente ‘rojo’ (Brassica juncea cv ‘rojo’) and arugula (Eruca sativa) Site-Specific Alternatives: o Columbus: Barley o Deming: Mustard with wheat Barley Mustard Barley Mustard o Las Uvas: Barley COVER CROP MEASUREMENTS CARRYOVER EFFECTS ON WEED SEEDBANK • Biomass at mustard termination • Weed biomass at mustard termination Mustard Cover Crop 100 No Cover Crop 60 • Glucosinolate content (pesticidal component of A B mustard cover crop) 80 40 CARRYOVER EFFECTS ON WEED SEEDBANKS 60 • Palmer amaranth seed persistence in buried packets 40 • Germination of persistent Palmer amaranth seeds 20 (laboratory) Germination Seed persistence 20 (% of seeds (% of buried) (% of persistent(% seeds) 0 0 Columbus Deming Leyendecker Columbus Deming Leyendecker Figure 2. -

Building Big Flavor
BUILDING BIG FLAVOR Watching your sodium intake? Balancing flavors is the key to a flavorful meal when reducing the salt in a dish. Think about adding these flavor enhancers instead of reaching for the salt shaker! Sweet Bitter Acidic Umami (Savory) Brings balance and Balances sweetness Brings brightness Makes a dish savory or roundness to a dish by and cuts richness - and adds a salty meaty tasting and balancing acidity and best used as flavor that enhances flavors - reach bitterness and background flavor balances for these before salt! highlighting other flavors sweetness Fruit juices, Nectars, Greens (Kale, Lemon, Lime, Tomato Products Concentrates, Chard, Dandelion, Orange and (especially canned, like Reductions, Caramelized Chicory, Watercress, Pineapple Juice, paste) Soy Sauce, Onions, Carrots, Sweet Arugula) Broccoli Vinegars, Wine, Mushrooms (especially Potatoes, Butternut Rabe, Broccoli, Tamarind, dried) Cured or brined Squash, Roasted Cabbage, Brussels Pickled Foods, foods (olives) Seaweed, Peppers, Honey, Maple Sprouts, Asparagus, Cranberries, Sour Fish Sauce, Fermented Syrup, Molasses, Dried Some Mustards, Cherries, Tomato Foods (Miso, Fermented Fruits, Tomato Paste, Grapefruit, Citrus Products Black beans, Sauerkraut) Beets, Reduced Vinegars, Rind/Zest, Beer, Aged cheeses (Parmesan, Wine, Wine, Teas (black & Blue, Gouda) Liquid Amino green) Acids, Seafood (especially dried), Worcestershire Sauce, Anchovy, Beef, Pork (especially cured), Chicken Don’t forget to read nutrition labels and watch for foods that are commonly high -

Tolerance of Vegetable Crops to Salinity M.C
Scientia Horticulturae 78 (1999) 5±38 Tolerance of vegetable crops to salinity M.C. Shannon*, C.M. Grieve U.S. Salinity Laboratory, Department of Agriculture, Agricultural Research Service, 450 W. Big Springs Road, Riverside, CA 92507, USA Abstract Global constraints on fresh water supplies and the need to dispose of agricultural, municipal, and industrial waste waters have intensified interest in water reuse options. In many instances, the value of the water is decreased solely because of its higher salt concentration. Although quantitative information on crop salt tolerance exists for over 130 crop species, there are many vegetables which lack definitive data. Vegetable crops are defined as herbaceous species grown for human consumption in which the edible portions consist of leaves, roots, hypocotyls, stems, petioles, and flower buds. The salt tolerance of vegetable species is important because the cash value of vegetables is usually high compared to field crops. In this review some general information is presented on how salinity affects plant growth and development and how different measurements of salinity in solution cultures, sand cultures, and field studies can be reconciled to a common basis. The salt tolerance of vegetables has been condensed and reported in a uniform format based on the best available data. Discrepancies and inconsistencies exist in some of the information due to differences in cultivars, environments, and experimental conditions. For a great number of species little or no useful information exists and there is an obvious need for research. Published by Elsevier Science B.V. Keywords: Salt tolerance; Ion composition Contents 1. Introduction ............................................................ 7 1.1. -

Identification of a Scar Marker Linked to a Shattering Resistance Trait in Sesame
Turk J Field Crops 2017, 22(2), 258-265 DOI: 10.17557/tjfc.359707 IDENTIFICATION OF A SCAR MARKER LINKED TO A SHATTERING RESISTANCE TRAIT IN SESAME Chalermpol PHUMICHAI1*, Weerachai MATTHAYATTHAWORN1, Nipha CHUENPOM1, Arunee WONGKAEW1, Phakaked SOMSAENG1, Tanapong YODYINGYONG1, Pherawich, PANKLANG1, Sujin JENWEERAWAT1, Yaowamarn KEAWSAARD1, Thitaporn PHUMICHAI2, Tanee SREEWONGCHAI1, Rangsarid KAVEETA1 1Kasetsart University, Faculty of Agriculture, Department of Agronomy, Bangkok, THAILAND. 2Rubber Research Institute of Thailand, Bangkok, THAILAND. *Corresponding author: [email protected] Received: 09.08.2017 ABSTRACT Sesame (Sesamum indicum L.) is one of the most important oil crops in temperate and tropical regions and is grown worldwide over an area of 5179 (hg ha-1) to produce 5.469.024 tonnes of seed. Capsule shattering before or during harvest can cause yield losses of greater than 50%. The objectives of the present study were to evaluate the inheritance of resistance to capsule shattering in the F2 derived from a cross between Cplus1, a sesame line with shattering-resistant capsules (Sh1Sh1Sh2Sh2), and KUAOX25, a line with shattering- susceptible capsules (sh1sh1sh2sh2-); and use bulked segregant analysis and Amplified Fragment Length Polymorphisms (AFLPs) to identify Sequence Characterized Amplified Region (SCAR) markers associated with shattering resistance. After screening 192 AFLP primer combinations, nine polymorphic bands were identified, and one of these AFLPs was developed into a Si-SR-32-19 SCAR that could distinguish between shattering-susceptible and shattering-resistant phenotypes. Keywords: AFLP, BSA, SCAR, Sesamum indicum L., shattering resistance INTRODUCTION production of sesame is estimated at 5.469.024 tonnes, and the average annual yield worldwide is roughly 5179 Sesame (Sesamum indicum L.), a member of the (hg ha-1) (FAOSTAT, 2014) Pedaliaceae family, is an important oilseed crop that is widely cultivated in tropical and subtropical areas (Ashri, The production of sesame is limited due to capsule 2010). -

21 CFR Ch. I (4–1–10 Edition) § 582.20
§ 582.20 21 CFR Ch. I (4–1–10 Edition) Common name Botanical name of plant source Marjoram, sweet .......................................................................... Majorana hortensis Moench. Mustard, black or brown .............................................................. Brassica nigra (L.) Koch. Mustard, brown ............................................................................ Brassica juncea (L.) Coss. Mustard, white or yellow .............................................................. Brassica hirta Moench. Nutmeg ........................................................................................ Myristica fragrans Houtt. Oregano (oreganum, Mexican oregano, Mexican sage, origan) Lippia spp. Paprika ......................................................................................... Capsicum annuum L. Parsley ......................................................................................... Petroselinum crispum (Mill.) Mansf. Pepper, black ............................................................................... Piper nigrum L. Pepper, cayenne ......................................................................... Capsicum frutescens L. or Capsicum annuum L. Pepper, red .................................................................................. Do. Pepper, white ............................................................................... Piper nigrum L. Peppermint .................................................................................. Mentha piperita L. Poppy seed -

Illinois Exotic Species List
Exotic Species in Illinois Descriptions for these exotic species in Illinois will be added to the Web page as time allows for their development. A name followed by an asterisk (*) indicates that a description for that species can currently be found on the Web site. This list does not currently name all of the exotic species in the state, but it does show many of them. It will be updated regularly with additional information. Microbes viral hemorrhagic septicemia Novirhabdovirus sp. West Nile virus Flavivirus sp. Zika virus Flavivirus sp. Fungi oak wilt Ceratocystis fagacearum chestnut blight Cryphonectria parasitica Dutch elm disease Ophiostoma novo-ulmi and Ophiostoma ulmi late blight Phytophthora infestans white-nose syndrome Pseudogymnoascus destructans butternut canker Sirococcus clavigignenti-juglandacearum Plants okra Abelmoschus esculentus velvet-leaf Abutilon theophrastii Amur maple* Acer ginnala Norway maple Acer platanoides sycamore maple Acer pseudoplatanus common yarrow* Achillea millefolium Japanese chaff flower Achyranthes japonica Russian knapweed Acroptilon repens climbing fumitory Adlumia fungosa jointed goat grass Aegilops cylindrica goutweed Aegopodium podagraria horse chestnut Aesculus hippocastanum fool’s parsley Aethusa cynapium crested wheat grass Agropyron cristatum wheat grass Agropyron desertorum corn cockle Agrostemma githago Rhode Island bent grass Agrostis capillaris tree-of-heaven* Ailanthus altissima slender hairgrass Aira caryophyllaea Geneva bugleweed Ajuga genevensis carpet bugleweed* Ajuga reptans mimosa -
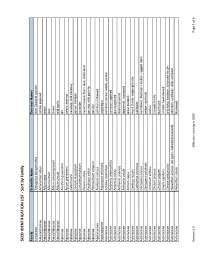
SEED IDENTIFICATION LIST - Sort by Family
SEED IDENTIFICATION LIST - Sort by Family Family Scientific Name Common Names Aizoaceae Tetragonia tetragonoides New Zealand spinach Amaranthaceae Amaranthus albus tumble pigweed Amaryllidaceae Allium cepa onion Amaryllidaceae Allium porrum leek Amaryllidaceae Allium schoenoprasum chives Amaryllidaceae Allium vineale wild garlic Apiaceae Anethum graveolens dill Apiaceae Apium graveolens celery, celeriac Apiaceae Carum carvi caraway; wild caraway Apiaceae Conium maculatum poison hemlock Apiaceae Coriandrum sativum coriander Apiaceae Daucus carota carrot; Queen Ane's lace; wild carrot Apiaceae Pastinaca sativa parsnip; wild parsnip Apiaceae Petroselinum crispum parsley Apocynaceae Asclepias syriaca common milkweed Asparagaceae Asparagus officinalis asparagus Asteraceae Achillea millefolium common yarrow, woolly yarrow Asteraceae Ambrosia artemisiifolia common ragweed Asteraceae Ambrosia trifida giant ragweed Asteraceae Anthemis arvensis field chamomile Asteraceae Anthemis cotula dogfennel, mayweed Asteraceae Arctium lappa great burdock Asteraceae Carduus nutans musk thistle, nodding thistle Asteraceae Carthamus tinctorius safflower Asteraceae Centaurea cyanus cornflower, bachelor's button, ragged robin Asteraceae Centaurea solstitialis yellow starthistle Asteraceae Cichorium endivia endive Asteraceae Cirsium arvense Canada thistle Asteraceae Cirsium vulgare bull thistle Asteraceae Crepis capillaris smooth hawksbeard Asteraceae Cynara cardunculus artichoke, cardoon, artichoke thistle Asteraceae Helianthus annuus (all types, cultivated and -

Antibacterial Activity of Different Plant and Callus Extracts a Comparative Study
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 2, ISSUE 10, OCTOBER 2013 ISSN 2277-8616 Antibacterial Activity Of Different Plant And Callus Extracts A Comparative Study V.D. Jadhav, S. M. Bhanuwanshe, S. P. Patil, D.V. Chaudhari, M.B. Adke Abstract: Current study includes antibacterial activity of different plant and callus extract such as Momordica charantia (Karela), Cucurbita pepo (Pumpkin), Capsicum annum (Chilli), Coriander sativum (Coriander), Brassica nigra (Mustard), Nigella sativa (Black Cumin), Trigonella foenum (Fenugreek) against eight pathogenic bacteria of which five are gram positive such as Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Streptococcus sp, Bacillus megaterium and three are gram negative such as Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa. Plant materials were extracted using ethanol. All the extracts showed zone of inhibition against these pathogenic bacteria which were tested by agar well diffusion method. The leaf extract of Momordica charantia, Nigella sativa, Barassica nigra showed good zone of inhibition as compared to other extracts against S.aureus (13mm, 17mm,11mm), E.coli (13mm,10mm,18mm), P. aeruginosa (11mm,12mm,10mm), Streptocococcus (14mm, 15mm, 10mm), B.subtilis (10mm,17mm,10mm), P. vulgaris (15mm,18mm, 10mm), B.cereus (13mm,16mm,12mm), B.megaterium (12mm,15mm,10mm). Explants from in vitro grown plants were cultured on MS-Medium with different combination of IAA and Kinetin for callus induction. Among those combination of IAA and Kinetin (0.1 x 0.0), (1.0 x 0.0), (0.4 x 0.5), (1.0 x 0.5), (1.5 x 0.5), (0.4 x 1.0), (0.8 x1.0), (0.1 x 1.5) mg/L have showed maximum growth of calli.