Phenolic Profiling of Veronica Spp. Grown in Mountain, Urban and Sand Soil Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sideritis Clandestina (Bory & Chaub.) Hayek; Sideritis Raeseri Boiss
2 February 2016 EMA/HMPC/39455/2015 Committee on Herbal Medicinal Products (HMPC) Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba Final Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use) Herbal substances (binomial scientific name of Sideritis scardica Griseb.; Sideritis clandestina the plant, including plant part) (Bory & Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba Herbal preparation Comminuted herbal substance Pharmaceutical form Comminuted herbal substance as herbal tea for oral use Rapporteur I. Chinou Peer-reviewer B. Kroes 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2016. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ................................................................................................................... 2 1. Introduction ....................................................................................................................... 4 1.1. Description of the herbal substance(s), herbal preparation(s) or combinations thereof .. 4 1.2. Search and assessment methodology ..................................................................... 8 2. Data on -

Shilin Yang Doctor of Philosophy
PHYTOCHEMICAL STUDIES OF ARTEMISIA ANNUA L. THESIS Presented by SHILIN YANG For the Degree of DOCTOR OF PHILOSOPHY of the UNIVERSITY OF LONDON DEPARTMENT OF PHARMACOGNOSY THE SCHOOL OF PHARMACY THE UNIVERSITY OF LONDON BRUNSWICK SQUARE, LONDON WC1N 1AX ProQuest Number: U063742 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U063742 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENT I wish to express my sincere gratitude to Professor J.D. Phillipson and Dr. M.J.O’Neill for their supervision throughout the course of studies. I would especially like to thank Dr. M.F.Roberts for her great help. I like to thank Dr. K.C.S.C.Liu and B.C.Homeyer for their great help. My sincere thanks to Mrs.J.B.Hallsworth for her help. I am very grateful to the staff of the MS Spectroscopy Unit and NMR Unit of the School of Pharmacy, and the staff of the NMR Unit, King’s College, University of London, for running the MS and NMR spectra. -

Herbal Principles in Cosmetics Properties and Mechanisms of Action Traditional Herbal Medicines for Modern Times
Traditional Herbal Medicines for Modern Times Herbal Principles in Cosmetics Properties and Mechanisms of Action Traditional Herbal Medicines for Modern Times Each volume in this series provides academia, health sciences, and the herbal medicines industry with in-depth coverage of the herbal remedies for infectious diseases, certain medical conditions, or the plant medicines of a particular country. Series Editor: Dr. Roland Hardman Volume 1 Shengmai San, edited by Kam-Ming Ko Volume 2 Rasayana: Ayurvedic Herbs for Rejuvenation and Longevity, by H.S. Puri Volume 3 Sho-Saiko-To: (Xiao-Chai-Hu-Tang) Scientific Evaluation and Clinical Applications, by Yukio Ogihara and Masaki Aburada Volume 4 Traditional Medicinal Plants and Malaria, edited by Merlin Willcox, Gerard Bodeker, and Philippe Rasoanaivo Volume 5 Juzen-taiho-to (Shi-Quan-Da-Bu-Tang): Scientific Evaluation and Clinical Applications, edited by Haruki Yamada and Ikuo Saiki Volume 6 Traditional Medicines for Modern Times: Antidiabetic Plants, edited by Amala Soumyanath Volume 7 Bupleurum Species: Scientific Evaluation and Clinical Applications, edited by Sheng-Li Pan Traditional Herbal Medicines for Modern Times Herbal Principles in Cosmetics Properties and Mechanisms of Action Bruno Burlando, Luisella Verotta, Laura Cornara, and Elisa Bottini-Massa Cover art design by Carlo Del Vecchio. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2010 by Taylor and Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-13: 978-1-4398-1214-3 (Ebook-PDF) This book contains information obtained from authentic and highly regarded sources. -

Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study
RESEARCH ARTICLE Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study Konstantinos M. Kasiotis1*, Pelagia Anastasiadou1, Antonis Papadopoulos2, Kyriaki Machera1* 1 Benaki Phytopathological Institute, Department of Pesticides Control and Phytopharmacy, Laboratory of Pesticides' Toxicology, Kifissia, Athens, Greece, 2 Benaki Phytopathological Institute, Department of Phytopathology, Laboratory of Non-Parasitic Diseases, Kifissia, Athens, Greece * [email protected] (KMK); [email protected] (KM) Abstract a1111111111 Propolis is a bee product that has been extensively used in alternative medicine and recently a1111111111 a1111111111 has gained interest on a global scale as an essential ingredient of healthy foods and cosmet- a1111111111 ics. Propolis is also considered to improve human health and to prevent diseases such as a1111111111 inflammation, heart disease, diabetes and even cancer. However, the claimed effects are anticipated to be correlated to its chemical composition. Since propolis is a natural product, its composition is consequently expected to be variable depending on the local flora align- ment. In this work, we present the development of a novel HPLC-PDA-ESI/MS targeted OPEN ACCESS method, used to identify and quantify 59 phenolic compounds in Greek propolis hydroalco- holic extracts. Amongst them, nine phenolic compounds are herein reported for the first time Citation: Kasiotis KM, Anastasiadou P, Papadopoulos A, Machera K (2017) Revisiting in Greek propolis. Alongside GC-MS complementary analysis was employed, unveiling Greek Propolis: Chromatographic Analysis and eight additional newly reported compounds. The antioxidant activity study of the propolis Antioxidant Activity Study. PLoS ONE 12(1): samples verified the potential of these extracts to effectively scavenge radicals, with the e0170077. doi:10.1371/journal.pone.0170077 extract of Imathia region exhibiting comparable antioxidant activity to that of quercetin. -

Distribution of Flavonoids Among Malvaceae Family Members – a Review
Distribution of flavonoids among Malvaceae family members – A review Vellingiri Vadivel, Sridharan Sriram, Pemaiah Brindha Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University, Thanjavur, Tamil Nadu, India Abstract Since ancient times, Malvaceae family plant members are distributed worldwide and have been used as a folk remedy for the treatment of skin diseases, as an antifertility agent, antiseptic, and carminative. Some compounds isolated from Malvaceae members such as flavonoids, phenolic acids, and polysaccharides are considered responsible for these activities. Although the flavonoid profiles of several Malvaceae family members are REVIEW REVIEW ARTICLE investigated, the information is scattered. To understand the chemical variability and chemotaxonomic relationship among Malvaceae family members summation of their phytochemical nature is essential. Hence, this review aims to summarize the distribution of flavonoids in species of genera namely Abelmoschus, Abroma, Abutilon, Bombax, Duboscia, Gossypium, Hibiscus, Helicteres, Herissantia, Kitaibelia, Lavatera, Malva, Pavonia, Sida, Theobroma, and Thespesia, Urena, In general, flavonols are represented by glycosides of quercetin, kaempferol, myricetin, herbacetin, gossypetin, and hibiscetin. However, flavonols and flavones with additional OH groups at the C-8 A ring and/or the C-5′ B ring positions are characteristic of this family, demonstrating chemotaxonomic significance. Key words: Flavones, flavonoids, flavonols, glycosides, Malvaceae, phytochemicals INTRODUCTION connate at least at their bases, but often forming a tube around the pistils. The pistils are composed of two to many connate he Malvaceae is a family of flowering carpels. The ovary is superior, with axial placentation, with plants estimated to contain 243 genera capitate or lobed stigma. The flowers have nectaries made with more than 4225 species. -

BIOLOGICAL PROPERTIES of SELECTED FLAVONOIDS of ROOIBOS (Aspalathus Linearis)
BIOLOGICAL PROPERTIES OF SELECTED FLAVONOIDS OF ROOIBOS (Aspalathus linearis) Petra W Snijman Thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Chemistry at the University of the Western Cape Study Leader: Prof IR Green Co-study Leaders: Prof E Joubert Prof WCA Gelderblom June 2007 ii DECLARATION I, the undersigned, hereby declare that the work contained in this thesis is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. _______________________________ ____________ Petra Wilhelmina Snijman Date Copyright © 2007 University of the Western Cape All rights reserved iii ABSTRACT Bioactivity-guided fractionation was used to identify the most potent antioxidant and antimutagenic fractions contained in the methanol extract of unfermented rooibos (Aspalathus linearis), as well as the bioactive principles for the most potent antioxidant fractions. The different extracts and fractions were screened using Salmonella typhimurium tester strain TA98 and metabolically activated 2- acetoaminofluorene (2-AAF) to evaluate antimutagenic potential, while the antioxidant potency was assessed by two different in vitro assays, i.e. the inhibition of Fe(II) induced microsomal lipid peroxidation and the scavenging of the 2,2'- azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation. The most polar XAD fraction displayed the most protection against 2-AAF induced mutagenesis in TA98. Successive fractionation of the two XAD fractions -

Phytochemical Studies of the Moss Species Plagiomnium Elatum and Plagiomnium Cuspidatum
journ. Hattori Bot. Lab. No. 67: 377- 382 (Dec. 1989) PHYTOCHEMICAL STUDIES OF THE MOSS SPECIES PLAGIOMNIUM ELATUM AND PLAGIOMNIUM CUSPIDATUM 1 S. ANHUT , T. SEEGER 1, J. B IEHL 1 , H. D . ZINSMEISTER 1 AND H . GEIGER 2 ABSTRACT. The flavonoid pattern of P/agiomnium cuspidatum and P. e/atum was evaluated. Fifteen dif ferent flavones, mainly flavone glycosides were isolated. The new natural compounds Elatin; 5-0H-Amento flavone; 2,3-Dihydro-5' -hydroxyamentoflavone, 2,3-Dihydro-5' -hydroxyrobustaflavone and 2,3-Dihydro- 5',3"'-dihydroxyamentoflavone were amongst them. The phytochemical relevance of these results is briefly discussed. INTRODUCTION The fi rst phytochemical studies on Mnium species was done by Kozlowski (1921). He detected Saponarin in Mnium cu:,pidatum, based on a chemical reaction with iodinepotassium iodide solution. Melchert & Alston (1965) reported on the occurrence of flavon C-glycosides in Mnium affine, McClure and Miller (1967) offlavonoids in Mnium cu.lpidatum. Koponen and Nilsson (1977) compared the flavonoid patterns of Plagiomnium elfipticum, P. medium, P. insigne, P. affine, P. tezukae, P. ciliare, P. ela/um, P. drummondii, P. japonicum, P. acutum and P. cuspidatum. Vandekerkhove (1977) isolated two f1avonoids from Mnium undulatum and characterized them as saponarin and an apigenin-6,8 di-C-glycoside. Further C- and C-O-glycosides from the same species were isolated and identified by Osterdahl (1979). Freitag et at. (1986) isolated two new isoorientin-O-diglycosides from P. affine. According to the "Generic revision of Mniaceae" (Koponen, 1968) all mentioned species were allocated to the genus Plagiomnium. The revised classification of the family Mniaceae resulted in four tribes with ten genera on the basis of morphological and karyological data. -

Serbian Journal of Experimental and Clinical Research Vol12
Editor-in-Chief Slobodan Janković Co-Editors Nebojša Arsenijević, Miodrag Lukić, Miodrag Stojković, Milovan Matović, Slobodan Arsenijević, Nedeljko Manojlović, Vladimir Jakovljević, Mirjana Vukićević Board of Editors Ljiljana Vučković-Dekić, Institute for Oncology and Radiology of Serbia, Belgrade, Serbia Dragić Banković, Faculty for Natural Sciences and Mathematics, University of Kragujevac, Kragujevac, Serbia Zoran Stošić, Medical Faculty, University of Novi Sad, Novi Sad, Serbia Petar Vuleković, Medical Faculty, University of Novi Sad, Novi Sad, Serbia Philip Grammaticos, Professor Emeritus of Nuclear Medicine, Ermou 51, 546 23, Th essaloniki, Macedonia, Greece Stanislav Dubnička, Inst. of Physics Slovak Acad. Of Sci., Dubravska cesta 9, SK-84511 Bratislava, Slovak Republic Luca Rosi, SAC Istituto Superiore di Sanita, Vaile Regina Elena 299-00161 Roma, Italy Richard Gryglewski, Jagiellonian University, Department of Pharmacology, Krakow, Poland Lawrence Tierney, Jr, MD, VA Medical Center San Francisco, CA, USA Pravin J. Gupta, MD, D/9, Laxminagar, Nagpur – 440022 India Winfried Neuhuber, Medical Faculty, University of Erlangen, Nuremberg, Germany Editorial Staff Ivan Jovanović, Gordana Radosavljević, Nemanja Zdravković Vladislav Volarević Management Team Snezana Ivezic, Milan Milojevic, Bojana Radojevic, Ana Miloradovic Corrected by Scientifi c Editing Service “American Journal Experts” Design PrstJezikIostaliPsi - Miljan Nedeljković Print Medical Faculty, Kragujevac Indexed in EMBASE/Excerpta Medica, Index Copernicus, BioMedWorld, KoBSON, -
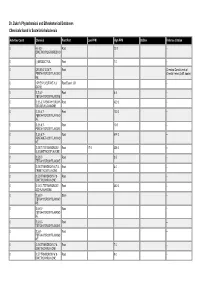
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals Found in Scutellaria Baicalensis
Dr. Duke's Phytochemical and Ethnobotanical Databases Chemicals found in Scutellaria baicalensis Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 (+)-5,5'- Root 22.0 -- DIMETHOXYLARICIRESINO L 0 (-)-ERODICTYOL Root 7.0 -- 0 (2R,3R)-2',3,5,6',7- Root Chemical Constituents of PENTAHYDROXYFLAVONO Oriental Herbs (3 diff. books) NE 0 1-PHENYL-BUTANE-1,3- Root Essent. Oil -- DIONE 0 2',3',5,7- Root 6.4 -- TETRAHYDROXYFLAVONE 0 2',3,5,6',7-PENTAHYDROXY- Root 420.0 -- 2(R),3(R)-FLAVANONE 0 2',3,5,6',7- Root 120.0 -- PENTAHYDROXYFLAVANO NE 0 2',3,5,6',7- Root 10.0 -- PENTAHYDROXYFLAVONE 0 2',3,5,6',7- Root 674.0 -- PENTAMETHOXYFLAVANO NE 0 2',5,5',7-TETRAHYDROXY- Root 17.0 356.0 -- 6',8-DIMETHOXYFLAVONE 0 2',5,5',7- Root 3.5 -- TETRAHYDROXYFLAVONE 0 2',5,5'-TRIHYDROXY-6,7,8- Root 4.0 -- TRIMETHOXYFLAVONE 0 2',5,5'-TRIHYDROXY-7,8- Root -- DIMETHOXYFLAVONE 0 2',5,6',7-TETRAHYDROXY- Root 380.0 -- 2(S)-FLAVANONE 0 2',5,6',7- Stem -- TETRAHYDROXYFLAVANO NE 0 2',5,6',7- Root -- TETRAHYDROXYFLAVANO NE 0 2',5,6',7- Root -- TETRAHYDROXYFLAVONE 0 2',5,6'- Root -- TETRAHYDROXYFLAVANO NE 0 2',5,6'-TRIHYDROXY-7,8- Root 7.0 -- DIMETHOXYFLAVONE 0 2',5,7-TRIHYDROXY-6',8- Root 9.0 -- DIMETHOXYFLAVONE Activities Count Chemical Plant Part Low PPM High PPM StdDev Refernce Citation 0 2',5,7-TRIHYDROXY-6',8- Root 7.0 -- TRIMETHOXYFLAVONE 0 2',5,7-TRIHYDROXY-6'- Root 4.0 -- METHOXYFLAVONE 0 2',5,7-TRIHYDROXY-8- Root 6.0 -- METHOXYFLAVONE 0 2',5,7- Root 6.0 -- TRIHYDROXYFLAVONE 0 2',5,8-TRIHYDROXY-6,7- Root 200.0 -- DIMETHOXYFLAVONE 0 2'- Root -- METHYLSKULLCAPFLAVO NE 0 2(S),2',5,6',7- Root 230.0 -- TETRAHYDROXYFLAVANO NE 0 2,2',4',6-TETRAHYDROXY- Root 5.0 -- 6'-METHOXYCHALCONE 1 2,3-DIHYDROBENZOFURAN Root Essent. -

RSC Advances
RSC Advances This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. This Accepted Manuscript will be replaced by the edited, formatted and paginated article as soon as this is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/advances Page 1 of 26 RSC Advances RSC Advances RSC Publishing REVIEW The chemistry and biological activities of natural products from Northern African plant families: From Cite this: DOI: 10.1039/x0xx00000x Aloaceae to Cupressaceae Fidele Ntie-Kang, a,b †* and Joseph N. Yongb†* Received 00th January 2014, Accepted 00th January 2014 Traditional medicinal practices play a key role in health care systems in countries with DOI: 10.1039/x0xx00000x developing economies. The aim of this survey was to validate the use of traditional medicine within Northern African communities. In this review, we summarize the ethnobotanical uses of www.rsc.org/advances selected plant species from the Northern African flora and attempt to correlate the activities of the isolated bioactive principles with known local uses of the plant species in traditional medicine. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0323402 A1 Ono Et Al
US 201003234O2A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0323402 A1 Ono et al. (43) Pub. Date: Dec. 23, 2010 (54) UDP-GLUCURONYL TRANSFERASE AND Publication Classification POLYNUCLEOTDE ENCODING THE SAME (51) Int. Cl. CI2P 9/60 (2006.01) (75) Inventors: Eiichiro Ono, Osaka (JP); Akio C7H 2L/04 (2006.01) Noguchi, Osaka (JP); Yuko Fukui, C7H 2L/00 (2006.01) Osaka (JP); Masako Mizutani, CI2N 9/10 (2006.01) Osaka (JP) CI2N 15/63 (2006.01) CI2N I/2 (2006.01) Correspondence Address: (52) U.S. Cl. ......... 435/75:536/23.2:536/23.1; 435/193; GREENBLUM & BERNSTEIN, P.L.C. 1950 ROLAND CLARKE PLACE 435/320.1; 435/252.33 RESTON, VA 20191 (US) (57) ABSTRACT The present invention provides a novel UDP-glucuronosyl (73) Assignee: SUNTORY HOLDINGS transferase and a polynucleotide encoding the same (for LIMITED, Osaka (JP) example, a polynucleotide comprising a polynucleotide con sisting of one nucleotide sequence selected from the group (21) Appl. No.: 12/678,161 consisting of the nucleotide sequence at positions 1 to 1359 in the nucleotide sequence represented by SEQ ID NO: 4, the (22) PCT Fled: Sep. 29, 2008 nucleotide sequence at positions 1 to 1365 in the nucleotide PCT NO.: PCT/UP2008/O67613 sequence represented by SEQ ID NO: 10, the nucleotide (86) sequence at positions 1 to 1371 in the nucleotide sequence S371 (c)(1), represented by SEQID NO: 12, and the nucleotide sequence (2), (4) Date: May 12, 2010 at positions 1 to 1371 in the nucleotide sequence represented by SEQ ID NO: 22; or a polynucleotide comprising a poly (30) Foreign Application Priority Data nucleotide encoding a protein having one amino acid sequence selected from the group consisting of SEQID NOS: Oct. -

Exudate Flavonoids in Some Gnaphalieae and Inuleae (Asteraceae) Eckhard Wollenwebera,*, Matthias Christa, R
Exudate Flavonoids in Some Gnaphalieae and Inuleae (Asteraceae) Eckhard Wollenwebera,*, Matthias Christa, R. Hugh Dunstanb, James N. Roitmanc, and Jan F. Stevensd a Institut für Botanik der TU Darmstadt, Schnittspahnstrasse 4, D-64287 Darmstadt, Germany. Fax: 0049-6151/164630. E-mail: [email protected] b School of Environmental and Life Sciences, The University of Newcastle, Callaghan, NSW 2308, Australia c Western Regional Research Center, USDA-ARS, 800 Buchanan Street, Albany, CA 94710, U.S.A. d Department of Pharmaceutical Sciences, 203 Pharmacy Building, Oregon State University, Corvallis, OR 97331, U.S.A. * Author for correspondance and reprint requests Z. Naturforsch. 60c, 671Ð678 (2005); received May 19, 2005 Three members of the tribe Gnaphalieae and six members of the tribe Inuleae (Astera- ceae) were analyzed for their exudate flavonoids. Whereas some species exhibit rather trivial flavonoids, others produce rare compounds. Spectral data of rare flavonoids are reported and their structural identification is discussed. 6-Oxygenation of flavonols is a common feature of two Inula species and Pulicaria sicula. By contrast, flavonoids with 8-oxygenation, but lacking 6-oxygenation, are common in two out of three Gnaphalieae species examined. In addition, B-ring deoxyflavonoids are abundantly present in the leaf exudates of Helichrysum italicum (Gnaphalieae). These distinctive features of the two Asteraceae tribes are in agreement with previous flavonoid surveys of these and related taxa. Key words: Gnaphalieae, Inuleae, Flavonoids Introduction in the flowering stage between October 1997 and Plants belonging to the sunflower family are August 2004. Inula britannica L. was collected in well-known to produce a wealth of flavonoid agly- August 2000 on the bank of the river Elbe near cones (Bohm and Stuessy, 2001).