Table S2. List of the Hits Kinases Chosen for Validation and Their Known Functions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplemental Information to Mammadova-Bach Et Al., “Laminin Α1 Orchestrates VEGFA Functions in the Ecosystem of Colorectal Carcinogenesis”
Supplemental information to Mammadova-Bach et al., “Laminin α1 orchestrates VEGFA functions in the ecosystem of colorectal carcinogenesis” Supplemental material and methods Cloning of the villin-LMα1 vector The plasmid pBS-villin-promoter containing the 3.5 Kb of the murine villin promoter, the first non coding exon, 5.5 kb of the first intron and 15 nucleotides of the second villin exon, was generated by S. Robine (Institut Curie, Paris, France). The EcoRI site in the multi cloning site was destroyed by fill in ligation with T4 polymerase according to the manufacturer`s instructions (New England Biolabs, Ozyme, Saint Quentin en Yvelines, France). Site directed mutagenesis (GeneEditor in vitro Site-Directed Mutagenesis system, Promega, Charbonnières-les-Bains, France) was then used to introduce a BsiWI site before the start codon of the villin coding sequence using the 5’ phosphorylated primer: 5’CCTTCTCCTCTAGGCTCGCGTACGATGACGTCGGACTTGCGG3’. A double strand annealed oligonucleotide, 5’GGCCGGACGCGTGAATTCGTCGACGC3’ and 5’GGCCGCGTCGACGAATTCACGC GTCC3’ containing restriction site for MluI, EcoRI and SalI were inserted in the NotI site (present in the multi cloning site), generating the plasmid pBS-villin-promoter-MES. The SV40 polyA region of the pEGFP plasmid (Clontech, Ozyme, Saint Quentin Yvelines, France) was amplified by PCR using primers 5’GGCGCCTCTAGATCATAATCAGCCATA3’ and 5’GGCGCCCTTAAGATACATTGATGAGTT3’ before subcloning into the pGEMTeasy vector (Promega, Charbonnières-les-Bains, France). After EcoRI digestion, the SV40 polyA fragment was purified with the NucleoSpin Extract II kit (Machery-Nagel, Hoerdt, France) and then subcloned into the EcoRI site of the plasmid pBS-villin-promoter-MES. Site directed mutagenesis was used to introduce a BsiWI site (5’ phosphorylated AGCGCAGGGAGCGGCGGCCGTACGATGCGCGGCAGCGGCACG3’) before the initiation codon and a MluI site (5’ phosphorylated 1 CCCGGGCCTGAGCCCTAAACGCGTGCCAGCCTCTGCCCTTGG3’) after the stop codon in the full length cDNA coding for the mouse LMα1 in the pCIS vector (kindly provided by P. -

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

Targeted Kinase Inhibitor Profiling Using a Hybrid Quadrupole-Orbitrap Mass Spectrometer
Targeted Kinase Inhibitor Profiling Note Application Using a Hybrid Quadrupole-Orbitrap Mass Spectrometer 1 2 3 Scott Peterman , Ryan D. Bomgarden , Rosa Viner 574 1Thermo Fisher Scientific, Cambridge, MA;2 Thermo Fisher Scientific, Rockford, IL; 3Thermo Fisher Scientific, San Jose, CA Key Words An emerging technology for identifying kinase inhibitor Q Exactive, targeted peptide quantification, msxSIM, kinome profiling by targets is based on chemical proteomic profiling of kinase MS, desthiobiotin nucleotide probes inhibitor specificity and binding affinity. This technology combines mass spectrometry (MS)-based identification Goal and quantitation with small molecule probe binding and To identify and quantify kinase inhibition by staurosporine using kinase enrichment to determine kinase active site occupancy active sites probes in combination with targeted, multiplexed SIM (msxSIM). during inhibition. One of these methods uses novel biotinylated ATP and/or ADP probes that irreversibly react with conserved lysine residues of kinase ATP binding Introduction sites.1,2 Selective enrichment of active-site peptides from Protein kinases are key enzymes involved in a wide array labeled kinase digests dramatically reduces background of complex cellular functions and pathways. Misregulation matrix and increases signal for MS analysis of low- or mutation of protein kinases underlies numerous disease abundance kinase peptides. Using this method, more than states, including tumorigenesis, making them ideal candidates 400 different protein and lipid kinases from various for drug development. However, identifying specific mammalian tissues and cell lines have been identified and kinase inhibitors is challenging due to the high degree of functionally assayed using targeted acquisition on an ion homology among subfamily members of the 500+ human trap mass spectrometer.1 The assays are available kinases. -

RASSF1A Interacts with and Activates the Mitotic Kinase Aurora-A
Oncogene (2008) 27, 6175–6186 & 2008 Macmillan Publishers Limited All rights reserved 0950-9232/08 $32.00 www.nature.com/onc ORIGINAL ARTICLE RASSF1A interacts with and activates the mitotic kinase Aurora-A L Liu1, C Guo1, R Dammann2, S Tommasi1 and GP Pfeifer1 1Division of Biology, Beckman Research Institute, City of Hope Cancer Center, Duarte, CA, USA and 2Institute of Genetics, University of Giessen, Giessen, Germany The RAS association domain family 1A (RASSF1A) gene tumorigenesis and carcinogen-induced tumorigenesis is located at chromosome 3p21.3 within a specific area of (Tommasi et al., 2005; van der Weyden et al., 2005), common heterozygous and homozygous deletions. RASS- supporting the notion that RASSF1A is a bona fide F1A frequently undergoes promoter methylation-asso- tumor suppressor. However, it is not fully understood ciated inactivation in human cancers. Rassf1aÀ/À mice how RASSF1A is involved in tumor suppression. are prone to both spontaneous and carcinogen-induced The biochemical function of the RASSF1A protein is tumorigenesis, supporting the notion that RASSF1A is a largely unknown. The homology of RASSF1A with the tumor suppressor. However, it is not fully understood how mammalian Ras effector novel Ras effector (NORE)1 RASSF1A is involved in tumor suppression pathways. suggests that the RASSF1A gene product may function Here we show that overexpression of RASSF1A inhibits in signal transduction pathways involving Ras-like centrosome separation. RASSF1A interacts with Aurora-A, proteins. However, recent data indicate that RASSF1A a mitotic kinase. Surprisingly, knockdown of RASS- itself binds to RAS only weakly and that binding to F1A by siRNA led to reduced activation of Aurora-A, RAS may require heterodimerization of RASSF1A and whereas overexpression of RASSF1A resulted in in- NORE1 (Ortiz-Vega et al., 2002). -

The Drug Sensitivity and Resistance Testing (DSRT) Approach
A phenotypic screening and machine learning platform eciently identifies triple negative breast cancer-selective and readily druggable targets Prson Gautam 1 Alok Jaiswal 1 Tero Aittokallio 1, 2 Hassan Al Ali 3 Krister Wennerberg 1,4 Identifying eective oncogenic targets is challenged by the complexity of genetic alterations in 1Institute for Molecular Medicine Finland (FIMM), HiLIFE, University of Helsinki, Finland cancer and their poorly understood relation to cell function and survival. There is a need for meth- Current kinome coverage of kinase inhibitors in TNBC exhibit diverse kinase dependencies MFM-223 is selectively addicted to FGFR2 2Department of Mathematics and Statistics, University of Turku, Finland 3The Miami Project to Cure Paralysis, Peggy and Harold Katz Family Drug Discovery Center, A A Sylvester Comprehensive Cancer Center, and Department of Neurological Surgery and Medicine ods that rapidly and accurately identify “pharmacologically eective” targets without the require- clinical evaluation TN Kinases MFM-223 CAL-120 MDA-MB-231 TNBC TNBC TNBC TNBC TNBC TNBC HER2+ 100 University of Miami Miller School of Medicine, Miami, FL 33136, USA. non- HER2+ FGFR1 0.97 0.00 0.00 MFM-223 BL1 BL2 M MSL IM LAR ER+, PR+ 50 ment for priori knowledge of complex signaling networks. We developed an approach that uses ma- cancerous FGFR2 56.46 0.00 0.00 CAL-120 25 4 MDA-MB-231 Biotech Research & Innovation Centre (BRIC) and Novo Nordisk Foundation Center HCC1937 CAL-85-1 CAL-120 MDA-MB-231 DU4475 CAL-148 MCF-10A SK-BR-3 BT-474 FGFR3 25.10 0.00 0.00 0 chine learning to relate results from unbiased phenotypic screening of kinase inhibitors to their bio- for Stem Cell Biology (DanStem), University of Copenhagen, Denmark HCC1599 HDQ-P1 BT-549 MDA-MB-436 MFM-223 FGFR4 0.00 0.00 0.00 MAXIS*Bk Clinical status MDA-MB-468 CAL-51 Hs578T MDA-MB-453 score chemical activity data. -

Anti-SEK1 / MKK4 Phospho (Ser80) Antibody (ARG51673)
Product datasheet [email protected] ARG51673 Package: 100 μl, 50 μl anti-SEK1 / MKK4 phospho (Ser80) antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes SEK1 / MKK4 phospho (Ser80) Tested Reactivity Hu, Ms, Rat Tested Application ICC/IF, IHC-P, WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name SEK1 / MKK4 Antigen Species Human Immunogen Peptide sequence around phosphorylation site of serine 80 (T-H-S(p)-I-E) derived from Human SEK1/MKK4. Conjugation Un-conjugated Alternate Names MEK 4; MAPK/ERK kinase 4; PRKMK4; SAPKK-1; SAPK/ERK kinase 1; SKK1; JNK-activating kinase 1; EC 2.7.12.2; MEK4; MAP kinase kinase 4; c-Jun N-terminal kinase kinase 1; SEK1; SAPKK1; MAPKK4; Stress- activated protein kinase kinase 1; JNKK1; MKK4; SERK1; SAPK kinase 1; Dual specificity mitogen- activated protein kinase kinase 4; JNKK; MAPKK 4 Application Instructions Application table Application Dilution ICC/IF 1:100 - 1:200 IHC-P 1:50 - 1:100 WB 1:500 - 1:1000 Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Calculated Mw 44 kDa Properties Form Liquid Purification Antibodies were produced by immunizing rabbits with KLH-conjugated synthetic phosphopeptide. Antibodies were purified by affinity-chromatography using epitope-specific phosphopeptide. In addition, non-phospho specific antibodies were removed by chromatogramphy using non- phosphopeptide. Buffer PBS (without Mg2+ and Ca2+, pH 7.4), 150mM NaCl, 0.02% Sodium azide and 50% Glycerol. Preservative 0.02% Sodium azide Stabilizer 50% Glycerol www.arigobio.com 1/3 Concentration 1 mg/ml Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. -

HIPK2 and Extrachromosomal Histone H2B Are Separately Recruited by Aurora-B for Cytokinesis
Oncogene https://doi.org/10.1038/s41388-018-0191-6 ARTICLE HIPK2 and extrachromosomal histone H2B are separately recruited by Aurora-B for cytokinesis 1 1 2 3 2 Laura Monteonofrio ● Davide Valente ● Manuela Ferrara ● Serena Camerini ● Roberta Miscione ● 3 1,2 1 Marco Crescenzi ● Cinzia Rinaldo ● Silvia Soddu Received: 14 November 2017 / Revised: 24 January 2018 / Accepted: 5 February 2018 © The Author(s) 2018. This article is published with open access Abstract Cytokinesis, the final phase of cell division, is necessary to form two distinct daughter cells with correct distribution of genomic and cytoplasmic materials. Its failure provokes genetically unstable states, such as tetraploidization and polyploidization, which can contribute to tumorigenesis. Aurora-B kinase controls multiple cytokinetic events, from chromosome condensation to abscission when the midbody is severed. We have previously shown that HIPK2, a kinase involved in DNA damage response and development, localizes at the midbody and contributes to abscission by phosphorylating extrachromosomal histone H2B at Ser14. Of relevance, HIPK2-defective cells do not phosphorylate H2B 1234567890();,: and do not successfully complete cytokinesis leading to accumulation of binucleated cells, chromosomal instability, and increased tumorigenicity. However, how HIPK2 and H2B are recruited to the midbody during cytokinesis is still unknown. Here, we show that regardless of their direct (H2B) and indirect (HIPK2) binding of chromosomal DNA, both H2B and HIPK2 localize at the midbody independently of nucleic acids. Instead, by using mitotic kinase-specific inhibitors in a spatio-temporal regulated manner, we found that Aurora-B kinase activity is required to recruit both HIPK2 and H2B to the midbody. -
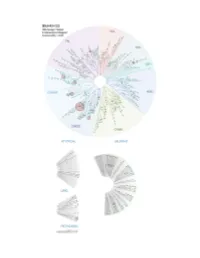
Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) BSJ-03-123 AAK1 AAK1 94 1000 BSJ-03-123 ABL1(E255K)-phosphorylated ABL1 79 1000 BSJ-03-123 ABL1(F317I)-nonphosphorylated ABL1 89 1000 BSJ-03-123 ABL1(F317I)-phosphorylated ABL1 98 1000 BSJ-03-123 ABL1(F317L)-nonphosphorylated ABL1 86 1000 BSJ-03-123 ABL1(F317L)-phosphorylated ABL1 89 1000 BSJ-03-123 ABL1(H396P)-nonphosphorylated ABL1 76 1000 BSJ-03-123 ABL1(H396P)-phosphorylated ABL1 90 1000 BSJ-03-123 ABL1(M351T)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1(Q252H)-nonphosphorylated ABL1 56 1000 BSJ-03-123 ABL1(Q252H)-phosphorylated ABL1 97 1000 BSJ-03-123 ABL1(T315I)-nonphosphorylated ABL1 100 1000 BSJ-03-123 ABL1(T315I)-phosphorylated ABL1 85 1000 BSJ-03-123 ABL1(Y253F)-phosphorylated ABL1 100 1000 BSJ-03-123 ABL1-nonphosphorylated ABL1 60 1000 BSJ-03-123 ABL1-phosphorylated ABL1 79 1000 BSJ-03-123 ABL2 ABL2 89 1000 BSJ-03-123 ACVR1 ACVR1 100 1000 BSJ-03-123 ACVR1B ACVR1B 95 1000 BSJ-03-123 ACVR2A ACVR2A 100 1000 BSJ-03-123 ACVR2B ACVR2B 96 1000 BSJ-03-123 ACVRL1 ACVRL1 84 1000 BSJ-03-123 ADCK3 CABC1 90 1000 BSJ-03-123 ADCK4 ADCK4 91 1000 BSJ-03-123 AKT1 AKT1 100 1000 BSJ-03-123 AKT2 AKT2 98 1000 BSJ-03-123 AKT3 AKT3 100 1000 BSJ-03-123 ALK ALK 100 1000 BSJ-03-123 ALK(C1156Y) ALK 78 1000 BSJ-03-123 ALK(L1196M) ALK 100 1000 BSJ-03-123 AMPK-alpha1 PRKAA1 93 1000 BSJ-03-123 AMPK-alpha2 PRKAA2 100 1000 BSJ-03-123 ANKK1 ANKK1 89 1000 BSJ-03-123 ARK5 NUAK1 98 1000 BSJ-03-123 ASK1 MAP3K5 100 1000 BSJ-03-123 ASK2 MAP3K6 92 1000 BSJ-03-123 AURKA -

Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) JNK-IN-8 AAK1 AAK1 69 1000 JNK-IN-8 ABL1(E255K)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317I)-nonphosphorylated ABL1 87 1000 JNK-IN-8 ABL1(F317I)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317L)-nonphosphorylated ABL1 65 1000 JNK-IN-8 ABL1(F317L)-phosphorylated ABL1 61 1000 JNK-IN-8 ABL1(H396P)-nonphosphorylated ABL1 42 1000 JNK-IN-8 ABL1(H396P)-phosphorylated ABL1 60 1000 JNK-IN-8 ABL1(M351T)-phosphorylated ABL1 81 1000 JNK-IN-8 ABL1(Q252H)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(Q252H)-phosphorylated ABL1 56 1000 JNK-IN-8 ABL1(T315I)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(T315I)-phosphorylated ABL1 92 1000 JNK-IN-8 ABL1(Y253F)-phosphorylated ABL1 71 1000 JNK-IN-8 ABL1-nonphosphorylated ABL1 97 1000 JNK-IN-8 ABL1-phosphorylated ABL1 100 1000 JNK-IN-8 ABL2 ABL2 97 1000 JNK-IN-8 ACVR1 ACVR1 100 1000 JNK-IN-8 ACVR1B ACVR1B 88 1000 JNK-IN-8 ACVR2A ACVR2A 100 1000 JNK-IN-8 ACVR2B ACVR2B 100 1000 JNK-IN-8 ACVRL1 ACVRL1 96 1000 JNK-IN-8 ADCK3 CABC1 100 1000 JNK-IN-8 ADCK4 ADCK4 93 1000 JNK-IN-8 AKT1 AKT1 100 1000 JNK-IN-8 AKT2 AKT2 100 1000 JNK-IN-8 AKT3 AKT3 100 1000 JNK-IN-8 ALK ALK 85 1000 JNK-IN-8 AMPK-alpha1 PRKAA1 100 1000 JNK-IN-8 AMPK-alpha2 PRKAA2 84 1000 JNK-IN-8 ANKK1 ANKK1 75 1000 JNK-IN-8 ARK5 NUAK1 100 1000 JNK-IN-8 ASK1 MAP3K5 100 1000 JNK-IN-8 ASK2 MAP3K6 93 1000 JNK-IN-8 AURKA AURKA 100 1000 JNK-IN-8 AURKA AURKA 84 1000 JNK-IN-8 AURKB AURKB 83 1000 JNK-IN-8 AURKB AURKB 96 1000 JNK-IN-8 AURKC AURKC 95 1000 JNK-IN-8 -

Gene Expression Profiling of Micrornas Associated with UCA1 in Bladder Cancer Cells
INTERNATIONAL JOURNAL OF ONCOLOGY 48: 1617-1627, 2016 Gene expression profiling of microRNAs associated with UCA1 in bladder cancer cells XIAOJUAN XIE1,2, JINGJING PAN1, LIQIANG WEI2, SHOUZHEN WU3, HUILIAN HOU4, XU LI3 and WEI CHEN1 1Department of Clinical Laboratory, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi 710061; 2Shaanxi Center for Clinical Laboratory, Shaanxi Provincial People's Hospital, Xi'an, Shaanxi 710068; 3Center for Translational Medicine, 4Department of Pathology, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi 710061, P.R. China Received November 11, 2015; Accepted December 27, 2015 DOI: 10.3892/ijo.2016.3357 Abstract. Emerging evidence indicates that non-coding and positively correlated with miR-196a, whereas UCA1 and RNAs, such as lncRNAs and microRNAs, play important miR-196a were negatively correlated with p27kip1, which was roles in diverse diseases, such as cancer, immune diseases downregulated in bladder cancer patients. Thus, our findings and cardiovascular diseases. Interestingly, lncRNAs could provided valuable information on miRNAs associated with directly or indirectly regulate the expression of miRNAs. UCA1 in bladder cancer, which could be helpful to further However, the expression profiling of miRNAs associated with explore the related genes and molecular networks fundamental UCA1 in bladder cancer remains unknown. Here, we used in bladder cancer progression. Illumina deep sequencing to sequence miRNA libraries from both the UCA1 knockdown and normal high-expression 5637 Introduction cells. We identified 225 and 235 miRNAs expressed in 5637 cells of normal high-expression and knockdown of UCA1, Recently, emerging studies have demonstrated that 70-90% of respectively. -

New Insights in RBM20 Cardiomyopathy
Current Heart Failure Reports (2020) 17:234–246 https://doi.org/10.1007/s11897-020-00475-x TRANSLATIONAL RESEARCH IN HEART FAILURE (J BACKS & M VAN DEN HOOGENHOF, SECTION EDITORS) New Insights in RBM20 Cardiomyopathy D. Lennermann1,2 & J. Backs1,2 & M. M. G. van den Hoogenhof1,2 Published online: 13 August 2020 # The Author(s) 2020 Abstract Purpose of Review This review aims to give an update on recent findings related to the cardiac splicing factor RNA-binding motif protein 20 (RBM20) and RBM20 cardiomyopathy, a form of dilated cardiomyopathy caused by mutations in RBM20. Recent Findings While most research on RBM20 splicing targets has focused on titin (TTN), multiple studies over the last years have shown that other splicing targets of RBM20 including Ca2+/calmodulin-dependent kinase IIδ (CAMK2D) might be critically involved in the development of RBM20 cardiomyopathy. In this regard, loss of RBM20 causes an abnormal intracellular calcium handling, which may relate to the arrhythmogenic presentation of RBM20 cardiomyopathy. In addition, RBM20 presents clinically in a highly gender-specific manner, with male patients suffering from an earlier disease onset and a more severe disease progression. Summary Further research on RBM20, and treatment of RBM20 cardiomyopathy, will need to consider both the multitude and relative contribution of the different splicing targets and related pathways, as well as gender differences. Keywords RBM20 . Dilated cardiomyopathy . CaMKIIδ . Calcium handling . Gender differences . Titin Introduction (ARVC), where a small number of genes account for most of the genetic causes, DCM-causing mutations have been ob- Dilated cardiomyopathy (DCM), as defined by left ventricular served in a variety of genes of diverse ontology [2]. -

TRIM67 Inhibits Tumor Proliferation and Metastasis by Mediating
Journal of Cancer 2020, Vol. 11 6025 Ivyspring International Publisher Journal of Cancer 2020; 11(20): 6025-6037. doi: 10.7150/jca.47538 Research Paper TRIM67 inhibits tumor proliferation and metastasis by mediating MAPK11 in Colorectal Cancer Ying Liu1*, Guiqi Wang1*, Xia Jiang1*, Wei Li1, Congjie Zhai1, Fangjian Shang1, Shihao Chen1, Zengren Zhao1 and Weifang Yu2 1. Department of General Surgery, Hebei Key Laboratory of Colorectal Cancer Precision Diagnosis and Treatment, The First Hospital of Hebei Medical University, Donggang Road No.89, Shijiazhuang, Hebei 050031, P.R. China. 2. Department of Endoscopy Center, The First Hospital of Hebei Medical University, Donggang Road No.89, Shijiazhuang, Hebei 050031, P.R. China. *These authors contributed equally to this work. Corresponding authors: Prof. Zengren Zhao or Weifang Yu, The First Hospital of Hebei Medical University, Donggang Road No.89, Shijiazhuang, Hebei 050031, P.R. China; Tel: +86 0311 85917217; E-mail: [email protected] or [email protected]. © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2020.04.28; Accepted: 2020.08.04; Published: 2020.08.18 Abstract Purpose: We recently reported that tripartite motif-containing 67 (TRIM67) activates p53 to suppress colorectal cancer (CRC). However, the function and mechanism of TRIM67 in the inhibition of CRC cell proliferation and metastasis remains to be further elucidated. Methods: We detected the expression of TRIM67 in CRC tissues compared with normal tissues and confirmed its relationship with clinicopathological features.