Biodiversity of Life Geological History of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systema Naturae∗
Systema Naturae∗ c Alexey B. Shipunov v. 5.802 (June 29, 2008) 7 Regnum Monera [ Bacillus ] /Bacteria Subregnum Bacteria [ 6:8Bacillus ]1 Superphylum Posibacteria [ 6:2Bacillus ] stat.m. Phylum 1. Firmicutes [ 6Bacillus ]2 Classis 1(1). Thermotogae [ 5Thermotoga ] i.s. 2(2). Mollicutes [ 5Mycoplasma ] 3(3). Clostridia [ 5Clostridium ]3 4(4). Bacilli [ 5Bacillus ] 5(5). Symbiobacteres [ 5Symbiobacterium ] Phylum 2. Actinobacteria [ 6Actynomyces ] Classis 1(6). Actinobacteres [ 5Actinomyces ] Phylum 3. Hadobacteria [ 6Deinococcus ] sed.m. Classis 1(7). Hadobacteres [ 5Deinococcus ]4 Superphylum Negibacteria [ 6:2Rhodospirillum ] stat.m. Phylum 4. Chlorobacteria [ 6Chloroflexus ]5 Classis 1(8). Ktedonobacteres [ 5Ktedonobacter ] sed.m. 2(9). Thermomicrobia [ 5Thermomicrobium ] 3(10). Chloroflexi [ 5Chloroflexus ] ∗Only recent taxa. Viruses are not included. Abbreviations and signs: sed.m. (sedis mutabilis); stat.m. (status mutabilis): s., aut i. (superior, aut interior); i.s. (incertae sedis); sed.p. (sedis possibilis); s.str. (sensu stricto); s.l. (sensu lato); incl. (inclusum); excl. (exclusum); \quotes" for environmental groups; * (asterisk) for paraphyletic taxa; / (slash) at margins for major clades (\domains"). 1Incl. \Nanobacteria" i.s. et dubitativa, \OP11 group" i.s. 2Incl. \TM7" i.s., \OP9", \OP10". 3Incl. Dictyoglomi sed.m., Fusobacteria, Thermolithobacteria. 4= Deinococcus{Thermus. 5Incl. Thermobaculum i.s. 1 4(11). Dehalococcoidetes [ 5Dehalococcoides ] 5(12). Anaerolineae [ 5Anaerolinea ]6 Phylum 5. Cyanobacteria [ 6Nostoc ] Classis 1(13). Gloeobacteres [ 5Gloeobacter ] 2(14). Chroobacteres [ 5Chroococcus ]7 3(15). Hormogoneae [ 5Nostoc ] Phylum 6. Bacteroidobacteria [ 6Bacteroides ]8 Classis 1(16). Fibrobacteres [ 5Fibrobacter ] 2(17). Chlorobi [ 5Chlorobium ] 3(18). Salinibacteres [ 5Salinibacter ] 4(19). Bacteroidetes [ 5Bacteroides ]9 Phylum 7. Spirobacteria [ 6Spirochaeta ] Classis 1(20). Spirochaetes [ 5Spirochaeta ] s.l.10 Phylum 8. Planctobacteria [ 6Planctomyces ]11 Classis 1(21). -

Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America North of Mexico with a Key to Species
Zootaxa 2835: 30–40 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America north of Mexico with a key to species J. E. McPHERSON1, RICHARD J. PACKAUSKAS2, ROBERT W. SITES3, STEVEN J. TAYLOR4, C. SCOTT BUNDY5, JEFFREY D. BRADSHAW6 & PAULA LEVIN MITCHELL7 1Department of Zoology, Southern Illinois University, Carbondale, Illinois 62901, USA. E-mail: [email protected] 2Department of Biological Sciences, Fort Hays State University, Hays, Kansas 67601, USA. E-mail: [email protected] 3Enns Entomology Museum, Division of Plant Sciences, University of Missouri, Columbia, Missouri 65211, USA. E-mail: [email protected] 4Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Illinois 61820, USA. E-mail: [email protected] 5Department of Entomology, Plant Pathology, & Weed Science, New Mexico State University, Las Cruces, New Mexico 88003, USA. E-mail: [email protected] 6Department of Entomology, University of Nebraska-Lincoln, Panhandle Research & Extension Center, Scottsbluff, Nebraska 69361, USA. E-mail: [email protected] 7Department of Biology, Winthrop University, Rock Hill, South Carolina 29733, USA. E-mail: [email protected] Abstract A review of Acanthocephala of America north of Mexico is presented with an updated key to species. A. confraterna is considered a junior synonym of A. terminalis, thus reducing the number of known species in this region from five to four. New state and country records are presented. Key words: Coreidae, Coreinae, Acanthocephalini, Acanthocephala, North America, review, synonymy, key, distribution Introduction The genus Acanthocephala Laporte currently is represented in America north of Mexico by five species: Acan- thocephala (Acanthocephala) declivis (Say), A. -

"Lophophorates" Brachiopoda Echinodermata Asterozoa
Deuterostomes Bryozoa Phoronida "lophophorates" Brachiopoda Echinodermata Asterozoa Stelleroidea Asteroidea Ophiuroidea Echinozoa Holothuroidea Echinoidea Crinozoa Crinoidea Chaetognatha (arrow worms) Hemichordata (acorn worms) Chordata Urochordata (sea squirt) Cephalochordata (amphioxoius) Vertebrata PHYLUM CHAETOGNATHA (70 spp) Arrow worms Fossils from the Cambrium Carnivorous - link between small phytoplankton and larger zooplankton (1-15 cm long) Pharyngeal gill pores No notochord Peculiar origin for mesoderm (not strictly enterocoelous) Uncertain relationship with echinoderms PHYLUM HEMICHORDATA (120 spp) Acorn worms Pharyngeal gill pores No notochord (Stomochord cartilaginous and once thought homologous w/notochord) Tornaria larvae very similar to asteroidea Bipinnaria larvae CLASS ENTEROPNEUSTA (acorn worms) Marine, bottom dwellers CLASS PTEROBRANCHIA Colonial, sessile, filter feeding, tube dwellers Small (1-2 mm), "U" shaped gut, no gill slits PHYLUM CHORDATA Body segmented Axial notochord Dorsal hollow nerve chord Paired gill slits Post anal tail SUBPHYLUM UROCHORDATA Marine, sessile Body covered in a cellulose tunic ("Tunicates") Filter feeder (» 200 L/day) - perforated pharnx adapted for filtering & repiration Pharyngeal basket contractable - squirts water when exposed at low tide Hermaphrodites Tadpole larvae w/chordate characteristics (neoteny) CLASS ASCIDIACEA (sea squirt/tunicate - sessile) No excretory system Open circulatory system (can reverse blood flow) Endostyle - (homologous to thyroid of vertebrates) ciliated groove -

Platyhelminthes, Nemertea, and "Aschelminthes" - A
BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. III - Platyhelminthes, Nemertea, and "Aschelminthes" - A. Schmidt-Rhaesa PLATYHELMINTHES, NEMERTEA, AND “ASCHELMINTHES” A. Schmidt-Rhaesa University of Bielefeld, Germany Keywords: Platyhelminthes, Nemertea, Gnathifera, Gnathostomulida, Micrognathozoa, Rotifera, Acanthocephala, Cycliophora, Nemathelminthes, Gastrotricha, Nematoda, Nematomorpha, Priapulida, Kinorhyncha, Loricifera Contents 1. Introduction 2. General Morphology 3. Platyhelminthes, the Flatworms 4. Nemertea (Nemertini), the Ribbon Worms 5. “Aschelminthes” 5.1. Gnathifera 5.1.1. Gnathostomulida 5.1.2. Micrognathozoa (Limnognathia maerski) 5.1.3. Rotifera 5.1.4. Acanthocephala 5.1.5. Cycliophora (Symbion pandora) 5.2. Nemathelminthes 5.2.1. Gastrotricha 5.2.2. Nematoda, the Roundworms 5.2.3. Nematomorpha, the Horsehair Worms 5.2.4. Priapulida 5.2.5. Kinorhyncha 5.2.6. Loricifera Acknowledgements Glossary Bibliography Biographical Sketch Summary UNESCO – EOLSS This chapter provides information on several basal bilaterian groups: flatworms, nemerteans, Gnathifera,SAMPLE and Nemathelminthes. CHAPTERS These include species-rich taxa such as Nematoda and Platyhelminthes, and as taxa with few or even only one species, such as Micrognathozoa (Limnognathia maerski) and Cycliophora (Symbion pandora). All Acanthocephala and subgroups of Platyhelminthes and Nematoda, are parasites that often exhibit complex life cycles. Most of the taxa described are marine, but some have also invaded freshwater or the terrestrial environment. “Aschelminthes” are not a natural group, instead, two taxa have been recognized that were earlier summarized under this name. Gnathifera include taxa with a conspicuous jaw apparatus such as Gnathostomulida, Micrognathozoa, and Rotifera. Although they do not possess a jaw apparatus, Acanthocephala also belong to Gnathifera due to their epidermal structure. ©Encyclopedia of Life Support Systems (EOLSS) BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. -

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final Report
Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final report Dr. Iryna Matsiakh Tbilisi 2014 This publication has been produced with the assistance of the European Union. The content, findings, interpretations, and conclusions of this publication are the sole responsibility of the FLEG II (ENPI East) Programme Team (www.enpi-fleg.org) and can in no way be taken to reflect the views of the European Union. The views expressed do not necessarily reflect those of the Implementing Organizations. CONTENTS LIST OF TABLES AND FIGURES ............................................................................................................................. 3 ABBREVIATIONS AND ACRONYMS ...................................................................................................................... 6 EXECUTIVE SUMMARY .............................................................................................................................................. 7 Background information ...................................................................................................................................... 7 Literature review ...................................................................................................................................................... 7 Methodology ................................................................................................................................................................. 8 Results and Discussion .......................................................................................................................................... -

A Multigene Phylogenetic Analysis of Terebelliform Annelids
Zhong et al. BMC Evolutionary Biology 2011, 11:369 http://www.biomedcentral.com/1471-2148/11/369 RESEARCH ARTICLE Open Access Detecting the symplesiomorphy trap: a multigene phylogenetic analysis of terebelliform annelids Min Zhong1, Benjamin Hansen2, Maximilian Nesnidal2, Anja Golombek2, Kenneth M Halanych1 and Torsten H Struck2* Abstract Background: For phylogenetic reconstructions, conflict in signal is a potential problem for tree reconstruction. For instance, molecular data from different cellular components, such as the mitochondrion and nucleus, may be inconsistent with each other. Mammalian studies provide one such case of conflict where mitochondrial data, which display compositional biases, support the Marsupionta hypothesis, but nuclear data confirm the Theria hypothesis. Most observations of compositional biases in tree reconstruction have focused on lineages with different composition than the majority of the lineages under analysis. However in some situations, the position of taxa that lack compositional bias may be influenced rather than the position of taxa that possess compositional bias. This situation is due to apparent symplesiomorphic characters and known as “the symplesiomorphy trap”. Results: Herein, we report an example of the sympleisomorphy trap and how to detect it. Worms within Terebelliformia (sensu Rouse & Pleijel 2001) are mainly tube-dwelling annelids comprising five ‘families’: Alvinellidae, Ampharetidae, Terebellidae, Trichobranchidae and Pectinariidae. Using mitochondrial genomic data, as well as data from the nuclear 18S, 28S rDNA and elongation factor-1a genes, we revealed incongruence between mitochondrial and nuclear data regarding the placement of Trichobranchidae. Mitochondrial data favored a sister relationship between Terebellidae and Trichobranchidae, but nuclear data placed Trichobranchidae as sister to an Ampharetidae/Alvinellidae clade. -

Plant Evolution an Introduction to the History of Life
Plant Evolution An Introduction to the History of Life KARL J. NIKLAS The University of Chicago Press Chicago and London CONTENTS Preface vii Introduction 1 1 Origins and Early Events 29 2 The Invasion of Land and Air 93 3 Population Genetics, Adaptation, and Evolution 153 4 Development and Evolution 217 5 Speciation and Microevolution 271 6 Macroevolution 325 7 The Evolution of Multicellularity 377 8 Biophysics and Evolution 431 9 Ecology and Evolution 483 Glossary 537 Index 547 v Introduction The unpredictable and the predetermined unfold together to make everything the way it is. It’s how nature creates itself, on every scale, the snowflake and the snowstorm. — TOM STOPPARD, Arcadia, Act 1, Scene 4 (1993) Much has been written about evolution from the perspective of the history and biology of animals, but significantly less has been writ- ten about the evolutionary biology of plants. Zoocentricism in the biological literature is understandable to some extent because we are after all animals and not plants and because our self- interest is not entirely egotistical, since no biologist can deny the fact that animals have played significant and important roles as the actors on the stage of evolution come and go. The nearly romantic fascination with di- nosaurs and what caused their extinction is understandable, even though we should be equally fascinated with the monarchs of the Carboniferous, the tree lycopods and calamites, and with what caused their extinction (fig. 0.1). Yet, it must be understood that plants are as fascinating as animals, and that they are just as important to the study of biology in general and to understanding evolutionary theory in particular. -

Number of Living Species in Australia and the World
Numbers of Living Species in Australia and the World 2nd edition Arthur D. Chapman Australian Biodiversity Information Services australia’s nature Toowoomba, Australia there is more still to be discovered… Report for the Australian Biological Resources Study Canberra, Australia September 2009 CONTENTS Foreword 1 Insecta (insects) 23 Plants 43 Viruses 59 Arachnida Magnoliophyta (flowering plants) 43 Protoctista (mainly Introduction 2 (spiders, scorpions, etc) 26 Gymnosperms (Coniferophyta, Protozoa—others included Executive Summary 6 Pycnogonida (sea spiders) 28 Cycadophyta, Gnetophyta under fungi, algae, Myriapoda and Ginkgophyta) 45 Chromista, etc) 60 Detailed discussion by Group 12 (millipedes, centipedes) 29 Ferns and Allies 46 Chordates 13 Acknowledgements 63 Crustacea (crabs, lobsters, etc) 31 Bryophyta Mammalia (mammals) 13 Onychophora (velvet worms) 32 (mosses, liverworts, hornworts) 47 References 66 Aves (birds) 14 Hexapoda (proturans, springtails) 33 Plant Algae (including green Reptilia (reptiles) 15 Mollusca (molluscs, shellfish) 34 algae, red algae, glaucophytes) 49 Amphibia (frogs, etc) 16 Annelida (segmented worms) 35 Fungi 51 Pisces (fishes including Nematoda Fungi (excluding taxa Chondrichthyes and (nematodes, roundworms) 36 treated under Chromista Osteichthyes) 17 and Protoctista) 51 Acanthocephala Agnatha (hagfish, (thorny-headed worms) 37 Lichen-forming fungi 53 lampreys, slime eels) 18 Platyhelminthes (flat worms) 38 Others 54 Cephalochordata (lancelets) 19 Cnidaria (jellyfish, Prokaryota (Bacteria Tunicata or Urochordata sea anenomes, corals) 39 [Monera] of previous report) 54 (sea squirts, doliolids, salps) 20 Porifera (sponges) 40 Cyanophyta (Cyanobacteria) 55 Invertebrates 21 Other Invertebrates 41 Chromista (including some Hemichordata (hemichordates) 21 species previously included Echinodermata (starfish, under either algae or fungi) 56 sea cucumbers, etc) 22 FOREWORD In Australia and around the world, biodiversity is under huge Harnessing core science and knowledge bases, like and growing pressure. -

The Plankton Lifeform Extraction Tool: a Digital Tool to Increase The
Discussions https://doi.org/10.5194/essd-2021-171 Earth System Preprint. Discussion started: 21 July 2021 Science c Author(s) 2021. CC BY 4.0 License. Open Access Open Data The Plankton Lifeform Extraction Tool: A digital tool to increase the discoverability and usability of plankton time-series data Clare Ostle1*, Kevin Paxman1, Carolyn A. Graves2, Mathew Arnold1, Felipe Artigas3, Angus Atkinson4, Anaïs Aubert5, Malcolm Baptie6, Beth Bear7, Jacob Bedford8, Michael Best9, Eileen 5 Bresnan10, Rachel Brittain1, Derek Broughton1, Alexandre Budria5,11, Kathryn Cook12, Michelle Devlin7, George Graham1, Nick Halliday1, Pierre Hélaouët1, Marie Johansen13, David G. Johns1, Dan Lear1, Margarita Machairopoulou10, April McKinney14, Adam Mellor14, Alex Milligan7, Sophie Pitois7, Isabelle Rombouts5, Cordula Scherer15, Paul Tett16, Claire Widdicombe4, and Abigail McQuatters-Gollop8 1 10 The Marine Biological Association (MBA), The Laboratory, Citadel Hill, Plymouth, PL1 2PB, UK. 2 Centre for Environment Fisheries and Aquacu∑lture Science (Cefas), Weymouth, UK. 3 Université du Littoral Côte d’Opale, Université de Lille, CNRS UMR 8187 LOG, Laboratoire d’Océanologie et de Géosciences, Wimereux, France. 4 Plymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK. 5 15 Muséum National d’Histoire Naturelle (MNHN), CRESCO, 38 UMS Patrinat, Dinard, France. 6 Scottish Environment Protection Agency, Angus Smith Building, Maxim 6, Parklands Avenue, Eurocentral, Holytown, North Lanarkshire ML1 4WQ, UK. 7 Centre for Environment Fisheries and Aquaculture Science (Cefas), Lowestoft, UK. 8 Marine Conservation Research Group, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. 9 20 The Environment Agency, Kingfisher House, Goldhay Way, Peterborough, PE4 6HL, UK. 10 Marine Scotland Science, Marine Laboratory, 375 Victoria Road, Aberdeen, AB11 9DB, UK. -

Solitary Entoprocts Living on Bryozoans - Commensals, Mutualists Or Parasites?
Journal of Experimental Marine Biology and Ecology 440 (2013) 15–21 Contents lists available at SciVerse ScienceDirect Journal of Experimental Marine Biology and Ecology journal homepage: www.elsevier.com/locate/jembe Solitary entoprocts living on bryozoans - Commensals, mutualists or parasites? Yuta Tamberg ⁎, Natalia Shunatova, Eugeniy Yakovis Department of Invertebrate Zoology, St. Petersburg State University, Universitetskaja nab. 7/9, St. Petersburg, 199034, Russian Federation article info abstract Article history: To assess the effects of interspecific interactions on community structure it is necessary to identify their sign. Received 24 December 2011 Interference in sessile benthic suspension-feeders is mediated by space and food. In the White Sea solitary Received in revised form 3 November 2012 entoproct Loxosomella nordgaardi almost restrictively inhabits the colonies of several bryozoans, including Accepted 7 November 2012 Tegella armifera. Since both entoprocts and their hosts are suspension-feeders, this strong spatial association Available online xxxx suggests feeding interference of an unknown sign. We mapped the colonies of T. armifera inhabited by entoprocts and examined stomachs of both species for Keywords: Bryozoa diatom shells. Distribution of L. nordgaardi was positively correlated with distribution of fully developed Commensalism and actively feeding polypides of T. armifera, i.e. areas of strong colony-wide currents. We compared diatom Entoprocta shells found in their stomachs and observed a diet overlap, especially in the size classes b15 μm. Size spectra Epibiosis of the diatom shells consumed by T. armifera and average number of diatom shells per gut were not affected Interactions by the presence of L. nordgaardi. According to these results, L. nordgaardi is a commensal of T. -
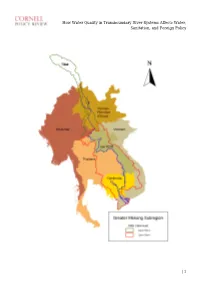
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy | 1 How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy David Tipping, 2001 By David C. Tipping Edited by Yeareen Yun Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any agency of the Australian government. Assumptions made within the analysis are not reflective of the position of any Australian government entity, or other organization or professional association. 1. INTRODUCTION Access to adequate water supply and sanitation is the core premise of local level water security. Effective management of transboundary river basin systems and water quality risks is therefore fundamental to social progress and quality of life. Improved water quality management benefits many individual lives in riparian nations, and, as demonstrated by the annual new year blessing of the fish migrations, society at large throughout the Mekong River Basin. In 2001, the author investigated the use of sustainable development indicators to improve the institutional effectiveness of international environmental management regimes. A new framework was designed to evaluate beneficial uses of water. In addition, a case study was developed on the Lower Mekong River Basin system, which integrated measures of water and environmental quality and socio-economic development. The research objectives were: (1) improving the understanding of water quality issues; (2) benchmarking water resources management performance at local, national and regional levels; and (3) enhancing technical and administrative capabilities of transboundary river basin management regimes through capacity development focused on the achievement of sustainable development objectives, and obligations and duties under international law.