Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

PRE Evaluation Report for Euonymus Alatus
PRE Evaluation Report -- Euonymus alatus Plant Risk Evaluator -- PRE™ Evaluation Report Euonymus alatus -- Texas 2017 Farm Bill PRE Project PRE Score: 16 -- Reject (high risk of invasiveness) Confidence: 71 / 100 Questions answered: 19 of 20 -- Valid (80% or more questions answered) Privacy: Public Status: Submitted Evaluation Date: September 21, 2017 This PDF was created on August 13, 2018 Page 1/18 PRE Evaluation Report -- Euonymus alatus Plant Evaluated Euonymus alatus Image by Chris Barton Page 2/18 PRE Evaluation Report -- Euonymus alatus Evaluation Overview A PRE™ screener conducted a literature review for this plant (Euonymus alatus) in an effort to understand the invasive history, reproductive strategies, and the impact, if any, on the region's native plants and animals. This research reflects the data available at the time this evaluation was conducted. Summary Euonymus alatus is naturalized across much of Eastern North America. It is a 1 to 4 meter tall shrub which an produce thickets in forest understory, displacing native vegetation. Seed production is prolific and dispersed by birds. General Information Status: Submitted Screener: Kim Taylor Evaluation Date: September 21, 2017 Plant Information Plant: Euonymus alatus If the plant is a cultivar, how does its behavior differs from its parent's? This evaluation is for the species, not a particular cultivar. Regional Information Region Name: Texas Page 3/18 PRE Evaluation Report -- Euonymus alatus Climate Matching Map To answer four of the PRE questions for a regional evaluation, a climate map with three climate data layers (Precipitation, UN EcoZones, and Plant Hardiness) is needed. These maps were built using a toolkit created in collaboration with GreenInfo Network, USDA, PlantRight, California-Invasive Plant Council, and The Information Center for the Environment at UC Davis. -

Scientific Support for Successful Implementation of the Natura 2000 Network
Scientific support for successful implementation of the Natura 2000 network Focus Area B Guidance on the application of existing scientific approaches, methods, tools and knowledge for a better implementation of the Birds and Habitat Directives Environment FOCUS AREA B SCIENTIFIC SUPPORT FOR SUCCESSFUL i IMPLEMENTATION OF THE NATURA 2000 NETWORK Imprint Disclaimer This document has been prepared for the European Commis- sion. The information and views set out in the handbook are Citation those of the authors only and do not necessarily reflect the Van der Sluis, T. & Schmidt, A.M. (2021). E-BIND Handbook (Part B): Scientific support for successful official opinion of the Commission. The Commission does not implementation of the Natura 2000 network. Wageningen Environmental Research/ Ecologic Institute /Milieu guarantee the accuracy of the data included. The Commission Ltd. Wageningen, The Netherlands. or any person acting on the Commission’s behalf cannot be held responsible for any use which may be made of the information Authors contained therein. Lead authors: This handbook has been prepared under a contract with the Anne Schmidt, Chris van Swaay (Monitoring of species and habitats within and beyond Natura 2000 sites) European Commission, in cooperation with relevant stakehold- Sander Mücher, Gerard Hazeu (Remote sensing techniques for the monitoring of Natura 2000 sites) ers. (EU Service contract Nr. 07.027740/2018/783031/ENV.D.3 Anne Schmidt, Chris van Swaay, Rene Henkens, Peter Verweij (Access to data and information) for evidence-based improvements in the Birds and Habitat Kris Decleer, Rienk-Jan Bijlsma (Guidance and tools for effective restoration measures for species and habitats) directives (BHD) implementation: systematic review and meta- Theo van der Sluis, Rob Jongman (Green Infrastructure and network coherence) analysis). -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Nematodes and Fungi) Against Curculio Nucum (Coleoptera: Curculionidae)
saqarTvelos mecnierebaTa erovnuli akademiis moambe, t. 15, #2, 2021 BULLETIN OF THE GEORGIAN NATIONAL ACADEMY OF SCIENCES, vol. 15, no. 2, 2021 Parasitology The Effectivenes of Entomopathogenic Microorganizsms (Nematodes and Fungi) Against Curculio nucum (Coleoptera: Curculionidae) Oleg Gorgadze*, Madona Kuchava*, Manana Lortkipanidze*, Nana Gratiashvili*, Medea Burjanadze** *Institute of Zoology, Ilia State University, Tbilisi, Georgia **Vasil Gulisashvili Forest Institute, Agricultural University of Georgia, Tbilisi (Presented by Academy Member Irakli Eliava) The nut weevil (NW), Curculio nucum, is one of the main pests of hazelnuts which damages nut buds, leaves and especially fruits. Sometimes it destroys 80-90 percent of the crop. The study presents the results of using entomopathogenic microorganisms (nematodes and fungi) against NW. Local entomopathogenic nematodes (EPN) Steinernema borjomiense, S. thesami, Heterorhabditis sp. and Boverin's fungi were used against this pest. The tests were carried out in laboratory and field conditions. Nematodes of this species were first used against NW. The tests were carried out separately and together with different doses of nematode concentracions (100, 50 and 25 IJs/insect) and Boverin – (2 g/L and 4 g/L). Among the nematodes used separately against insect pests, S. borjomiense and Heterorhabditis sp. (in a dose of 100 IJs/insect) showed the greatest effect (100%). As for the separate application of Boverin, it turned out to be less effective (42.5% of adults and 43.2% of larvae died). On insect pests, a much greater effect was observed when using combined preparations of nematodes and fungi in laboratory and field trials. In the laboratory, after the use of combined preparations, 100% death of harmful insects is recorded. -

Integration of Entomopathogenic Fungi Into IPM Programs: Studies Involving Weevils (Coleoptera: Curculionoidea) Affecting Horticultural Crops
insects Review Integration of Entomopathogenic Fungi into IPM Programs: Studies Involving Weevils (Coleoptera: Curculionoidea) Affecting Horticultural Crops Kim Khuy Khun 1,2,* , Bree A. L. Wilson 2, Mark M. Stevens 3,4, Ruth K. Huwer 5 and Gavin J. Ash 2 1 Faculty of Agronomy, Royal University of Agriculture, P.O. Box 2696, Dangkor District, Phnom Penh, Cambodia 2 Centre for Crop Health, Institute for Life Sciences and the Environment, University of Southern Queensland, Toowoomba, Queensland 4350, Australia; [email protected] (B.A.L.W.); [email protected] (G.J.A.) 3 NSW Department of Primary Industries, Yanco Agricultural Institute, Yanco, New South Wales 2703, Australia; [email protected] 4 Graham Centre for Agricultural Innovation (NSW Department of Primary Industries and Charles Sturt University), Wagga Wagga, New South Wales 2650, Australia 5 NSW Department of Primary Industries, Wollongbar Primary Industries Institute, Wollongbar, New South Wales 2477, Australia; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +61-46-9731208 Received: 7 September 2020; Accepted: 21 September 2020; Published: 25 September 2020 Simple Summary: Horticultural crops are vulnerable to attack by many different weevil species. Fungal entomopathogens provide an attractive alternative to synthetic insecticides for weevil control because they pose a lesser risk to human health and the environment. This review summarises the available data on the performance of these entomopathogens when used against weevils in horticultural crops. We integrate these data with information on weevil biology, grouping species based on how their developmental stages utilise habitats in or on their hostplants, or in the soil. -

Molecular Basis of Pheromonogenesis Regulation in Moths
Chapter 8 Molecular Basis of Pheromonogenesis Regulation in Moths J. Joe Hull and Adrien Fónagy Abstract Sexual communication among the vast majority of moths typically involves the synthesis and release of species-specifc, multicomponent blends of sex pheromones (types of insect semiochemicals) by females. These compounds are then interpreted by conspecifc males as olfactory cues regarding female reproduc- tive readiness and assist in pinpointing the spatial location of emitting females. Studies by multiple groups using different model systems have shown that most sex pheromones are synthesized de novo from acetyl-CoA by functionally specialized cells that comprise the pheromone gland. Although signifcant progress was made in identifying pheromone components and elucidating their biosynthetic pathways, it wasn’t until the advent of modern molecular approaches and the increased avail- ability of genetic resources that a more complete understanding of the molecular basis underlying pheromonogenesis was developed. Pheromonogenesis is regulated by a neuropeptide termed Pheromone Biosynthesis Activating Neuropeptide (PBAN) that acts on a G protein-coupled receptor expressed at the surface of phero- mone gland cells. Activation of the PBAN receptor (PBANR) triggers a signal trans- duction cascade that utilizes an infux of extracellular Ca2+ to drive the concerted action of multiple enzymatic steps (i.e. chain-shortening, desaturation, and fatty acyl reduction) that generate the multicomponent pheromone blends specifc to each species. In this chapter, we provide a brief overview of moth sex pheromones before expanding on the molecular mechanisms regulating pheromonogenesis, and con- clude by highlighting recent developments in the literature that disrupt/exploit this critical pathway. J. J. Hull (*) USDA-ARS, US Arid Land Agricultural Research Center, Maricopa, AZ, USA e-mail: [email protected] A. -

On Turkish Cerambyx Linnaeus, 1758 with Zoogeogrephical Remarks (Coleoptera: Cerambycidae: Cerambycinae)
_____________Mun. Ent. Zool. Vol. 4, No. 2, June 2009__________ 301 ON TURKISH CERAMBYX LINNAEUS, 1758 WITH ZOOGEOGREPHICAL REMARKS (COLEOPTERA: CERAMBYCIDAE: CERAMBYCINAE) Hüseyin Özdikmen* and Semra Turgut* * Gazi Üniversitesi, Fen-Edebiyat Fakültesi, Biyoloji Bölümü, 06500 Ankara / Türkiye. E- mails: [email protected] and [email protected] [Özdikmen, H. & Turgut, S. 2009. On Turkish Cerambyx Linnaeus, 1758 with zoogeogrephical remarks (Coleoptera: Cerambycidae: Cerambycinae). Munis Entomology & Zoology 4 (2): 301-319] ABSTRACT: All taxa of the genus Cerambyx Linnaeus, 1758 in Turkey and the whole world are evaluated. The genus is also discussed in detail. The main aim of this catalogic work is to clarify current status of the genus in Turkey. New faunistical data are given in the text. A key for Turkish Cerambyx species is also given in the text. KEY WORDS: Cerambyx, Cerambycinae, Cerambycini, Cerambycidae. Subfamily CERAMBYCINAE Latreille, 1802 Tribe CERAMBYCINI Latreille, 1802 = Cerambycina Latreille, 1804 = Cérambyçaires Mulsant, 1839 = Cérambycitae verae Thomson, 1860 = Cerambycites Fairmaire, 1864 = Cerambycina Thomson, 1866 = Cérambycides virais Lacordaire, 1869 = Sphallotrichina Martins & Monné, 2002 Type genus: Cerambyx Linnaeus, 1758 The size is large in general. It has brown reddish coloration, slightly brilliant. Head is more or less salient. It is projecting, with very pronounced furrows. Antennae are variable length and long in general, the segments are either smoothed and hornlike or flattened or streamlined. The eyes are large, strongly cut away. They present strong necklines and very rude facets. The maxillary palpus has triangular last segment; tongue membranous, strongly bilobed. The protorax is rugulose, in general it has conspicuous teeth laterally. It is wrinkled or pleated from side to side. -

Environmental Assessment Report: Georgia, Port of Poti
Environmental Assessment Report Environmental Audit Report September 2009 Prepared by Scientific Research Firm Gamma for Poti Sea Port Corporation This report has been submitted to ADB by Poti Sea Port Corporation and is made publicly available in accordance with ADB’s public communications policy (2005). It does not necessarily reflect the views of ADB. Poti Sea Port Corporation Environmental Audit Report for Current Operations of Poti Sea Port Executed by: Scientific Research Firm Gamma President Vakhtang Gvakharia Tbilisi-Poti 2009 9 M. Alexidze st, 0193, Tbilisi, Georgia tel: +(995 32) 330 274, 330 374 tel/fax +(995 32) 333 268 e-mail: [email protected] SCIENTIFIC RESEARCH FIRM GAMMA Environmental Audit, Poti Sea Port Corp. Page 2 of 38 Content 1 INTRODUCTION.............................................................................................................................................3 2 BRIEF DESCRIPTION OF POTI SEA PORT OPERATIONS ........................................................................4 2.1 ABOUT POTI SEA PORT .............................................................................................................................4 2.2 PORT’S EXTENSIVE DEVELOPMENT............................................................................................................7 3 DESCRIPTION OF PORT’S TECHNOLOGICAL PROCESSES .....................................................................7 3.1 TECHNOLOGICAL SCHEME AND CAPACITY OF OIL PRODUCT HANDLING ..................................................7 -
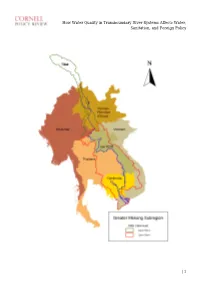
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy | 1 How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy David Tipping, 2001 By David C. Tipping Edited by Yeareen Yun Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any agency of the Australian government. Assumptions made within the analysis are not reflective of the position of any Australian government entity, or other organization or professional association. 1. INTRODUCTION Access to adequate water supply and sanitation is the core premise of local level water security. Effective management of transboundary river basin systems and water quality risks is therefore fundamental to social progress and quality of life. Improved water quality management benefits many individual lives in riparian nations, and, as demonstrated by the annual new year blessing of the fish migrations, society at large throughout the Mekong River Basin. In 2001, the author investigated the use of sustainable development indicators to improve the institutional effectiveness of international environmental management regimes. A new framework was designed to evaluate beneficial uses of water. In addition, a case study was developed on the Lower Mekong River Basin system, which integrated measures of water and environmental quality and socio-economic development. The research objectives were: (1) improving the understanding of water quality issues; (2) benchmarking water resources management performance at local, national and regional levels; and (3) enhancing technical and administrative capabilities of transboundary river basin management regimes through capacity development focused on the achievement of sustainable development objectives, and obligations and duties under international law. -

Cylindrocladium Buxicola Nom. Cons. Prop.(Syn. Calonectria
I Promotors: Prof. dr. ir. Monica Höfte Laboratory of Phytopathology, Department of Crop Protection Faculty of Bioscience Engineering Ghent University Dr. ir. Kurt Heungens Institute for Agricultural and Fisheries Research (ILVO) Plant Sciences Unit - Crop Protection Dean: Prof. dr. ir. Guido Van Huylenbroeck Rector: Prof. dr. Anne De Paepe II Bjorn Gehesquière Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences III Dutch translation of the title: Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) in Buxus: moleculaire karakterisering, epidemiologie, waardplantresistentie en chemische bestrijding. Please refer to this work as follows: Gehesquière B. (2014). Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control. Phd Thesis. Ghent University, Belgium The author and the promotors give authorisation to consult and to copy parts of this work for personal use only. Any other use is limited by Laws of Copyright. Permission to reproduce any material contained in this work should be obtained from the author. The promotors, The author, Prof. dr. ir. M. Höfte Dr. ir. K. Heungens ir. B. Gehesquière IV Een woordje van dank…. Dit dankwoord schrijven is ongetwijfeld het leukste onderdeel van deze thesis, en een mooie afsluiting van een interessante periode. Terugblikkend op de voorbije vier jaren kan ik enkel maar beamen dat een doctoraat zoveel meer is dan een wetenschappelijke uitdaging. Het is een levensreis in al zijn facetten, waarbij ik mezelf heb leren kennen in al mijn goede en slechte kantjes. -

Biodiversity of Wood-Decay Fungi in Italy
AperTO - Archivio Istituzionale Open Access dell'Università di Torino Biodiversity of wood-decay fungi in Italy This is the author's manuscript Original Citation: Availability: This version is available http://hdl.handle.net/2318/88396 since 2016-10-06T16:54:39Z Published version: DOI:10.1080/11263504.2011.633114 Terms of use: Open Access Anyone can freely access the full text of works made available as "Open Access". Works made available under a Creative Commons license can be used according to the terms and conditions of said license. Use of all other works requires consent of the right holder (author or publisher) if not exempted from copyright protection by the applicable law. (Article begins on next page) 28 September 2021 This is the author's final version of the contribution published as: A. Saitta; A. Bernicchia; S.P. Gorjón; E. Altobelli; V.M. Granito; C. Losi; D. Lunghini; O. Maggi; G. Medardi; F. Padovan; L. Pecoraro; A. Vizzini; A.M. Persiani. Biodiversity of wood-decay fungi in Italy. PLANT BIOSYSTEMS. 145(4) pp: 958-968. DOI: 10.1080/11263504.2011.633114 The publisher's version is available at: http://www.tandfonline.com/doi/abs/10.1080/11263504.2011.633114 When citing, please refer to the published version. Link to this full text: http://hdl.handle.net/2318/88396 This full text was downloaded from iris - AperTO: https://iris.unito.it/ iris - AperTO University of Turin’s Institutional Research Information System and Open Access Institutional Repository Biodiversity of wood-decay fungi in Italy A. Saitta , A. Bernicchia , S. P. Gorjón , E.