Assessment of Forest Pests and Diseases in Native Boxwood Forests of Georgia Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Damage Level of Cydalima Perspectalis (Lepidoptera: Crambidae) on Naturally Growing and Ornamental Box Populations in Artvin, Turkey
Kastamonu Uni., Orman Fakültesi Dergisi, 2019, 19 (2):144-151 Research Article Kastamonu Univ., Journal of Forestry Faculty Doi:10.17475/kastorman.626286 Damage Level of Cydalima perspectalis (Lepidoptera: Crambidae) on Naturally Growing and Ornamental Box Populations in Artvin, Turkey Hazan ALKAN AKINCI1* , Oğuz KURDOĞLU2 1Artvin Çoruh University, Faculty of Forestry, Artvin, TURKEY 2 Karadeniz Technical University, Faculty of Forestry, Trabzon, TURKEY *Corresponding Author: [email protected] Received Date: 29.11.2018 Accepted Date: 25.03.2019 Abstract Aim of study: The box tree moth, Cydalima perspectalis (Lepidoptera: Crambidae), is an important alien invasive species on box, Buxus sempervirens, in Turkey. It was first detected in 2011 in Istanbul. It is a native pest of box plants in Asia. Its first discovery in Europe has been made in 2007 in Germany. Since then it has been successfully established in various ecosystems in Europe. Caterpillars feed on box leaves and cause severe defoliation and tree deaths. In this study, damage level and defoliation percentage were investigated on ornamental and naturally growing box plants. Area of study: Box plants were sampled in Artvin in the Eastern Black Sea Region of Turkey. Material and Method: A total of 90 box plants that were either naturally growing or ornamental box plants were sampled Main results: Majority of the naturally growing box plants (63.4%) had strong and very strong damages, and 71.4% of the ornamental box plants had middle and strong damage levels. Research highlights: Of the all observed plants, 53.4% had 40-100% defoliation and 25% of these plants did not recover. -

Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final Report
Assessment of Forest Pests and Diseases in Protected Areas of Georgia Final report Dr. Iryna Matsiakh Tbilisi 2014 This publication has been produced with the assistance of the European Union. The content, findings, interpretations, and conclusions of this publication are the sole responsibility of the FLEG II (ENPI East) Programme Team (www.enpi-fleg.org) and can in no way be taken to reflect the views of the European Union. The views expressed do not necessarily reflect those of the Implementing Organizations. CONTENTS LIST OF TABLES AND FIGURES ............................................................................................................................. 3 ABBREVIATIONS AND ACRONYMS ...................................................................................................................... 6 EXECUTIVE SUMMARY .............................................................................................................................................. 7 Background information ...................................................................................................................................... 7 Literature review ...................................................................................................................................................... 7 Methodology ................................................................................................................................................................. 8 Results and Discussion .......................................................................................................................................... -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

Pest Alert: Box Tree Moth (Cydalima Perspectalis)
Pest Alert Box Tree Moth (Cydalima perspectalis) The box tree moth is an invasive pest that primarily feeds on boxwood species (Buxus spp). In its native range, it also feeds on burning bush (Euonymus alatus), Japanese spindletree (E. japonicus), purple holly (Ilex chinensis), and orange jessamine (Murraya paniculate) once boxwood in the vicinity are completely defoliated. Distribution and Spread The box tree moth is native to temperate and subtropical regions in Asia. It was first reported in Europe in 2007, after which it spread rapidly across European countries and into Western Asia and Northern Africa. In 2018, it was documented in Canada. The rate of spread for the box tree moth has varied since its introduction in Europe, with some cases peaking at 96 miles per year. Long distance movement of the box tree moth across Europe occurred primarily through the movement of infested Adult moths (top and bottom left), damage (bottom) boxwood plantings. Box tree moths are highly mobile and used for edging, as hedges, thick brown border spanning 1.6 and are good fliers. Natural spread and/or clipped into different shapes to 1.8 inches. Some adults have of this moth in Europe is about 3 to make topiaries. The box tree completely brown wings with a small to 6 miles per year. One analysis moth can cause heavy defoliation of white streak on each forewing. Males from Europe concluded that natural boxwood plants if populations are left and females show both colorations. dispersal from continental Europe unchecked. Defoliation of existing to the United Kingdom was possible, and new growth can kill the plant. -

First Record of the Box Tree Moth Cydalima Perspectalis (Walker, 1859) (Lepidoptera: Crambidae) in Lithuania
LIETUVOS ENTOMOLOGŲ DRAUGIJOS DARBAI. 2 (30) tomas 55 FIRST RECORD OF THE BOX TREE MOTH CYDALIMA PERSPECTALIS (WALKER, 1859) (LEPIDOPTERA: CRAMBIDAE) IN LITHUANIA BRIGITA PAULAVIČIŪTĖ1, DARIUS MIKALAUSKAS2 1Kaunas T. Ivanauskas Zoological Museum, Laisvės al. 106, LT-44253, Kaunas, Lithuania. 2 Lithuanian Entomological Society, Akademijos 2, LT-08412 Vilnius, Lithuania. E-mail of corresponding author: [email protected] Introduction Biological invasion by alien species is a great ecological and economical threat, with a multitude of negative impacts on human and animal health, local biodiversity (flora and fauna) and cultural landscape (Hulme & Roy, 2010). The box tree moth Cydalima perspectalis (Walker, 1859) (Lepidoptera: Crambidae) is an invasive species on box tree Buxus spp. in Europe, which has been spreading and establishing across the continent during the last decade. The pest was included in the alert list of the European Plant Protection Organisation (EPPO) in 2007 but was removed in 2011 (Strachinis et al., 2015). The box tree moth Cydalima perspectalis (Walker, 1859) is known from the humid subtropical regions of East Asia, India (Hampson, 1896), China (Walker, 1859), Japan (Inoue, 1982), Korea (Park, 2008), and Far East Russia (Kirpichnikova, 2005). The larvae feed on the leaves of Buxus microphylla Siebold &Zucc., but also accept other Buxus species (Maruyama, 1993). In 2006, the box tree moth was registered in southwestern Germany (Krüger, 2008). In 2007, it was also found in Switzerland (Billen, 2007) and -

2013, XLVIII: 27-37 Grădina Botanică “Alexandru Borza” Cluj-Napoca
Contribuţii Botanice – 2013, XLVIII: 27-37 Grădina Botanică “Alexandru Borza” Cluj-Napoca THE PRESENCE OF VERONICA SPECIES IN DIFFERENT NATURA 2000 HABITAT TYPES IN ROMANIA Silvia OROIAN,1 Mihaela SĂMĂRGHIŢAN2 1 University of Medicine and Pharmacy, Târgu-Mureş, Faculty of Pharmacy, Pharmaceutical Botany and Cell Biology Department, 38 Gh. Marinescu Street, RO-540139 Târgu-Mureş, Romania 2 Mureş County Museum, Natural Science Department, 24 Horea Street, RO-540036 Târgu-Mureş, Romania e-mail: [email protected] Abstract: A principal objective of this study was to summarise the distribution of Veronica species in Romania, where such an inventory has not existed before. Taxa of Veronica genus were identified in 311 plant associations and 36 subassociations framed in 132 alliances. Of the 311 plant associations, 138 are included in various types of NATURA 2000 Habitats. The review of phytosociological data reveals the presence of Veronica taxa in 42 types of Natura 2000 habitats with scientific significance. Some of these Veronica taxa are characteristic species for alliances (i.e. Veronicion baumgartenii Coldea 1991, Veronico officinalis-Quercion Pop 1971) or differential species at plant association level (Veronico baumgartenii-Saxifragetum bryoidis Boşcaiu et al. 1977). Keywords: Veronica genus, Natura 2000 habitats, plant associations Introduction Veronica is considered among the richest genera of the vascular flora of Romania as well as of other European countries. As regards their systematic position, Veronica species were included until recently in the Scrophularioideae (Antirrhinoideae) subfamily, Scrophulariaceae family, Scrophulariales order, Asterideae subclass, Magnoliopsida class [7]. Because there are several classification systems for this genus, here we mention three of them, which we consider most recent. -
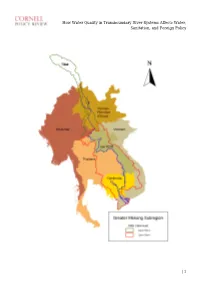
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy
How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy | 1 How Water Quality in Transboundary River Systems Affects Water, Sanitation, and Foreign Policy David Tipping, 2001 By David C. Tipping Edited by Yeareen Yun Disclaimer: The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any agency of the Australian government. Assumptions made within the analysis are not reflective of the position of any Australian government entity, or other organization or professional association. 1. INTRODUCTION Access to adequate water supply and sanitation is the core premise of local level water security. Effective management of transboundary river basin systems and water quality risks is therefore fundamental to social progress and quality of life. Improved water quality management benefits many individual lives in riparian nations, and, as demonstrated by the annual new year blessing of the fish migrations, society at large throughout the Mekong River Basin. In 2001, the author investigated the use of sustainable development indicators to improve the institutional effectiveness of international environmental management regimes. A new framework was designed to evaluate beneficial uses of water. In addition, a case study was developed on the Lower Mekong River Basin system, which integrated measures of water and environmental quality and socio-economic development. The research objectives were: (1) improving the understanding of water quality issues; (2) benchmarking water resources management performance at local, national and regional levels; and (3) enhancing technical and administrative capabilities of transboundary river basin management regimes through capacity development focused on the achievement of sustainable development objectives, and obligations and duties under international law. -

52 Southern Forest Insect Work Conference
Proceedings 52nd Southern Forest Insect Work Conference Gulfport July 28 – 31, 2009 Courtyard by Marriott Gulfport Beachfront Gulfport, Mississippi PROCEEDINGS 52nd Annual SOUTHERN FOREST INSECT WORK CONFERENCE Courtyard by Marriott Gulfport Beachfront Gulfport, Mississippi 28–31 July 2009 John Nowak, Program Chairman Andy Londo and John Riggins, Local Arrangements Officers: 2008–2009 Chairman ...................................................................................... Scott Salom (2007–2009) Secretary-Treasurer ........................................................................................ Will Shepherd Counselors..................................................................................... Laurie Reid (2005–2009) ....................................................................................... John Nowak (2007–2010) ....................................................................................... Andy Londo (2008–2012) ii TABLE OF CONTENTS Registration List ..................................................................................................................1 Group Pictures .....................................................................................................................2 Program ................................................................................................................................6 Minutes 2009 .....................................................................................................................26 Treasurer's Report .............................................................................................................31 -

Cylindrocladium Buxicola Nom. Cons. Prop.(Syn. Calonectria
I Promotors: Prof. dr. ir. Monica Höfte Laboratory of Phytopathology, Department of Crop Protection Faculty of Bioscience Engineering Ghent University Dr. ir. Kurt Heungens Institute for Agricultural and Fisheries Research (ILVO) Plant Sciences Unit - Crop Protection Dean: Prof. dr. ir. Guido Van Huylenbroeck Rector: Prof. dr. Anne De Paepe II Bjorn Gehesquière Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences III Dutch translation of the title: Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) in Buxus: moleculaire karakterisering, epidemiologie, waardplantresistentie en chemische bestrijding. Please refer to this work as follows: Gehesquière B. (2014). Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control. Phd Thesis. Ghent University, Belgium The author and the promotors give authorisation to consult and to copy parts of this work for personal use only. Any other use is limited by Laws of Copyright. Permission to reproduce any material contained in this work should be obtained from the author. The promotors, The author, Prof. dr. ir. M. Höfte Dr. ir. K. Heungens ir. B. Gehesquière IV Een woordje van dank…. Dit dankwoord schrijven is ongetwijfeld het leukste onderdeel van deze thesis, en een mooie afsluiting van een interessante periode. Terugblikkend op de voorbije vier jaren kan ik enkel maar beamen dat een doctoraat zoveel meer is dan een wetenschappelijke uitdaging. Het is een levensreis in al zijn facetten, waarbij ik mezelf heb leren kennen in al mijn goede en slechte kantjes. -

A Five-Gene Phylogeny of Pezizomycotina
Mycologia, 98(6), 2006, pp. 1018–1028. # 2006 by The Mycological Society of America, Lawrence, KS 66044-8897 A five-gene phylogeny of Pezizomycotina Joseph W. Spatafora1 Burkhard Bu¨del Gi-Ho Sung Alexandra Rauhut Desiree Johnson Department of Biology, University of Kaiserslautern, Cedar Hesse Kaiserslautern, Germany Benjamin O’Rourke David Hewitt Maryna Serdani Harvard University Herbaria, Harvard University, Robert Spotts Cambridge, Massachusetts 02138 Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331 Wendy A. Untereiner Department of Botany, Brandon University, Brandon, Franc¸ois Lutzoni Manitoba, Canada Vale´rie Hofstetter Jolanta Miadlikowska Mariette S. Cole Vale´rie Reeb 2017 Thure Avenue, St Paul, Minnesota 55116 Ce´cile Gueidan Christoph Scheidegger Emily Fraker Swiss Federal Institute for Forest, Snow and Landscape Department of Biology, Duke University, Box 90338, Research, WSL Zu¨ rcherstr. 111CH-8903 Birmensdorf, Durham, North Carolina 27708 Switzerland Thorsten Lumbsch Matthias Schultz Robert Lu¨cking Biozentrum Klein Flottbek und Botanischer Garten der Imke Schmitt Universita¨t Hamburg, Systematik der Pflanzen Ohnhorststr. 18, D-22609 Hamburg, Germany Kentaro Hosaka Department of Botany, Field Museum of Natural Harrie Sipman History, Chicago, Illinois 60605 Botanischer Garten und Botanisches Museum Berlin- Dahlem, Freie Universita¨t Berlin, Ko¨nigin-Luise-Straße Andre´ Aptroot 6-8, D-14195 Berlin, Germany ABL Herbarium, G.V.D. Veenstraat 107, NL-3762 XK Soest, The Netherlands Conrad L. Schoch Department of Botany and Plant Pathology, Oregon Claude Roux State University, Corvallis, Oregon 97331 Chemin des Vignes vieilles, FR - 84120 MIRABEAU, France Andrew N. Miller Abstract: Pezizomycotina is the largest subphylum of Illinois Natural History Survey, Center for Biodiversity, Ascomycota and includes the vast majority of filamen- Champaign, Illinois 61820 tous, ascoma-producing species. -

Acceptance and Suitability of the Box Tree Moth Cydalima Perspectalis As Host for the Tachinid Parasitoid Exorista Larvarum
Bulletin of Insectology 72 (1): 150-160, 2019 ISSN 1721-8861 eISSN 2283-0332 Acceptance and suitability of the box tree moth Cydalima perspectalis as host for the tachinid parasitoid Exorista larvarum Antonio MARTINI, Cinzia DI VITANTONIO, Maria Luisa DINDO Dipartimento di Scienze e Tecnologie Agro-Alimentari (DISTAL), Università di Bologna, Italy Abstract A laboratory bioassay and anatomical and histological studies were conducted to evaluate the acceptance and suitability of an ex- otic insect, the box tree moth Cydalima perspectalis (Walker) (Lepidoptera Crambidae), as host for the native parasitoid Exorista larvarum (L.) (Diptera Tachinidae). The factitious host Galleria mellonella (L.) (Lepidoptera Pyralidae) was maintained as con- trol. In the bioassay, C. perspectalis and G. mellonella mature larvae were separately exposed for 3 hours to E. larvarum mated females. Box tree moth larvae were accepted by E. larvarum females, but a lower number of eggs were laid on them than on G. mellonella. Most eggs hatched, as also shown in the anatomical and histological studies, but no puparia formed in any accepted C. perspectalis larva. Two out of six first instar E. larvarum larvae penetrated the body of a box tree moth larva and were encap- sulated. The encapsulation response turned into the formation of the respiratory funnel by two parasitoid larvae, similarly to what happens in G. mellonella. The results obtained in this study showed that the exotic species was unsuitable as host for E. larvarum. The mortality following the parasitoid larval activity (independently of successful parasitization) was, however, not significantly different between C. perspectalis and G. mellonella. The overall results suggest that the mortality of C. -

Pathogenicity and Molecular Detection of Nectriaceous Fungi Associated with Black Root Rot of Avocado
IX World Avocado Congress, 23 – 27 September, 2019, Medellín, Colombia WAC-130 PATHOGENICITY AND MOLECULAR DETECTION OF NECTRIACEOUS FUNGI ASSOCIATED WITH BLACK ROOT ROT OF AVOCADO L. E. Parkinson, D. P. Le, R. G. Shivas and E. K. Dann Dr Louisamarie Parkinson BBiotech(Hons), PhD Centre for Horticultural Science Queensland Alliance for Agriculture and Food Innovation (QAAFI) The University of Queensland, Brisbane Qld 4072 Australia [email protected] | https://www.qaafi.uq.edu.au THE UNIVERSITY OF QUEENSLAND St Lucia, Brisbane, Queensland Australia PATHOGENICITY AND MOLECULAR DETECTION OF NECTRIACEOUS FUNGI ASSOCIATED WITH BLACK ROOT ROT OF AVOCADO L. E. Parkinson1, D. P. Le1, R. G. Shivas2, E. K. Dann1 1 Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Australia 2Centre for Crop Health, The University of Southern Queensland, Australia KEY WORDS Calonectria, Calonectria ilicicola, Dactylonectria, Dactylonectria macrodidyma, diagnostic test, diversity, loop-mediated isothermal amplification (LAMP) SUMMARY Black root rot of avocado associated with soilborne nectriaceous fungi is an aggressive disease of nursery trees and young orchards transplants, causing tree stunting, wilt, severe root necrosis, rapid decline and death within a year after planting. This study aimed to identify the fungal genera associated with the disease, determine the causal agents of black root rot, and develop a rapid molecular test for detection of key pathogens in avocado roots. A disease survey in all Australian growing regions collected 153 nectriaceous fungal isolates from roots of 91 symptomatic and healthy avocado trees and other hosts including peanut, papaya, blueberry, custard apple and grapevine. The fungal isolates were identified with phylogenetic analyses of ITS, β-tubulin and Histone H3 sequenced genes.