Pathogenicity and Molecular Detection of Nectriaceous Fungi Associated with Black Root Rot of Avocado
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calonectria Ilicicola
OctoberPathogen of11 the month –October 2011 a b c d Reitsma & Fig. 1. Calonectria ilicicola; asci with ascospores (a); microsclerotia on a papaya root (b); conidium of anamorph, Cylindrocladium parasiticum (c); orange perithecia on papaya root (d); Photo credits M. Male (a,b,c), L. Vawdrey (d) Disease: Collar rot of papaya Pathogen: Calonectria ilicicola (anamorph: Cylindrocladium parasiticum) Boedijn Classification: K: Fungi, D: Ascomycota, C: Sordariomycetes, O: Hypocreales, F: Nectriaceae Calonectria ilicicola (Fig. 1) is a fungal pathogen found throughout the world infecting a wide variety of crops. It is best known as the causal agent of peg, pod and root necrosis of peanuts, collar rot of koa, leaf spot in some eucalypts and various root and collar rots of soybean, anthurium, groundnut and lucerne. In Australia, it causes black rot of peanut and collar rot of papaya. Its development is favoured by poorly drained soils. Host Range: Key Distinguishing Features: C. ilicicola is found throughout the world. It is In seedlings, the initial symptoms are of a discoloured pathogenic on a wide variety of plants including water soaked, root collar. As this rot develops, plants peanuts, soybean, anthurium, eucalypts, lucerne, are stunted and leaves become chlorotic and wilt. groundnut and papaya. Eventually, orange to red perithecia and thick-walled ilicicola brown microsclerotia will form on roots at or near the Impact: soil line (Fig 1 b,d). In culture, asci are clavate, 90- In far north Queensland, collar rot of papaya caused 140 x 12-19μm, tapering to a long thin stalk by C. ilicicola affects young plants in poorly drained containing eight hyaline ascospores (Fig1,a). -

Cylindrocladium Buxicola Nom. Cons. Prop.(Syn. Calonectria
I Promotors: Prof. dr. ir. Monica Höfte Laboratory of Phytopathology, Department of Crop Protection Faculty of Bioscience Engineering Ghent University Dr. ir. Kurt Heungens Institute for Agricultural and Fisheries Research (ILVO) Plant Sciences Unit - Crop Protection Dean: Prof. dr. ir. Guido Van Huylenbroeck Rector: Prof. dr. Anne De Paepe II Bjorn Gehesquière Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences III Dutch translation of the title: Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) in Buxus: moleculaire karakterisering, epidemiologie, waardplantresistentie en chemische bestrijding. Please refer to this work as follows: Gehesquière B. (2014). Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control. Phd Thesis. Ghent University, Belgium The author and the promotors give authorisation to consult and to copy parts of this work for personal use only. Any other use is limited by Laws of Copyright. Permission to reproduce any material contained in this work should be obtained from the author. The promotors, The author, Prof. dr. ir. M. Höfte Dr. ir. K. Heungens ir. B. Gehesquière IV Een woordje van dank…. Dit dankwoord schrijven is ongetwijfeld het leukste onderdeel van deze thesis, en een mooie afsluiting van een interessante periode. Terugblikkend op de voorbije vier jaren kan ik enkel maar beamen dat een doctoraat zoveel meer is dan een wetenschappelijke uitdaging. Het is een levensreis in al zijn facetten, waarbij ik mezelf heb leren kennen in al mijn goede en slechte kantjes. -

Epidemiological Studies on the Infection Process and Symptom Expression of Soybean Sudden Death Syndrome Carlos Cecilio Gongora-Canul Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2010 Epidemiological studies on the infection process and symptom expression of soybean sudden death syndrome Carlos Cecilio Gongora-canul Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Plant Pathology Commons Recommended Citation Gongora-canul, Carlos Cecilio, "Epidemiological studies on the infection process and symptom expression of soybean sudden death syndrome" (2010). Graduate Theses and Dissertations. 11510. https://lib.dr.iastate.edu/etd/11510 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Epidemiological studies on the infection process and symptom expression of soybean sudden death syndrome by Carlos Cecilio Góngora-Canul A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Pathology Program of Study Committee: Leonor Leandro, Major Professor Gary Munkvold Greg Tylka X. B Yang Dan Nordman Iowa State University Ames, Iowa 2010 Copyright © Carlos Cecilio Góngora-Canul, 2010. All rights reserved. ii DEDICATION To my Lord, for giving me the blessing and the adventure to live. To my mother Elsa and my father Elias for their endless love and to all my brothers and sisters (Javier, Roberto, Martha, Manuel, Enrique and Nicte-Há) for all their great love affection. -

Assessment of Forest Pests and Diseases in Native Boxwood Forests of Georgia Final Report
Assessment of Forest Pests and Diseases in Native Boxwood Forests of Georgia Final report Dr. Iryna Matsiakh Forestry Department, Ukrainian National Forestry University (Lviv) Tbilisi 2016 TABLE OF CONTENT LIST OF TABLES AND FIGURES .................................................................................................................................. 2 ABBREVIATIONS AND ACRONYMS ........................................................................................................................... 5 EXECUTIVE SUMMARY .................................................................................................................................................. 6 INTRODUCTION .............................................................................................................................................................. 10 1. BACKGROUND INFORMATION ............................................................................................................................ 11 1.1. Biodiversity of Georgia ........................................................................................................................................ 11 1.2. Forest Ecosystems .................................................................................................................................................. 12 1.3. Boxwood Forests in Forests Habitat Classification ................................................................................. 14 1.4. Georgian Forests Habitat in the Context of Climate Change -
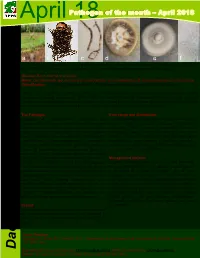
Dactylonectria Lombard & Crous
April Pathogen18 of the month – April 2018 a b c d e f Fig. 1. Black root rot symptoms in young orchard transplants (a), necrotic avocado roots (b, c) Dactylonectria macrodidyma on ½ sPDA at 3.75 cm after 10 days growth (d, e), D. macrodidyma macroconidia at 40 × magnification (f) Disease: Black root rot of avocado Name: Dactylonectria spp. including D. macrodidyma, D. novozelandica, D. pauciseptata and D. anthuriicola Classification: K: Fungi, D: Ascomycota, C: Sordariomycetes, O: Hypocreales, F: Nectriaceae Black root rot caused by nectriaceous fungi is a severe disease of avocado nursery trees and young orchard transplants, causing decline and death within one year of planting. Symptoms include stunting, wilt, leaf chlorosis and browning, leaf drop prior to tree death caused by severe necrosis of the root system. In Australia black root rot of avocado is caused by Calonectria ilicicola and several Dactylonectria spp. The Pathogen: Host range and distribution: Species of Dactylonectria (reported as Dactylonectria spp. cause root rot diseases in various Cylindrocarpon in older literature) have often been hosts including avocado (Persea americana), isolated from necrotic avocado roots. Dactylonectria grapevine (Vitis vinifera), cherimoya (Annona macrodidyma is the most prevalent of the pathogens cherimola), kiwifruit (Actinidia deliciosa) and olive found in symptomatic avocado roots. Dactylonectria (Olea europaea). Dactylonectria spp. associated with novozelandica, D. pauciseptata and D. anthuriicola avocado have been reported in Australia and Italy. have also been isolated from avocado roots and However the fungal genus is reported globally across Lombard & Crous shown to be pathogenic in glasshouse tests with numerous horticultural industries. seedlings. While Dactylonectria spp. -

(Hypocreales) Proposed for Acceptance Or Rejection
IMA FUNGUS · VOLUME 4 · no 1: 41–51 doi:10.5598/imafungus.2013.04.01.05 Genera in Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales) ARTICLE proposed for acceptance or rejection Amy Y. Rossman1, Keith A. Seifert2, Gary J. Samuels3, Andrew M. Minnis4, Hans-Josef Schroers5, Lorenzo Lombard6, Pedro W. Crous6, Kadri Põldmaa7, Paul F. Cannon8, Richard C. Summerbell9, David M. Geiser10, Wen-ying Zhuang11, Yuuri Hirooka12, Cesar Herrera13, Catalina Salgado-Salazar13, and Priscila Chaverri13 1Systematic Mycology & Microbiology Laboratory, USDA-ARS, Beltsville, Maryland 20705, USA; corresponding author e-mail: Amy.Rossman@ ars.usda.gov 2Biodiversity (Mycology), Eastern Cereal and Oilseed Research Centre, Agriculture & Agri-Food Canada, Ottawa, ON K1A 0C6, Canada 3321 Hedgehog Mt. Rd., Deering, NH 03244, USA 4Center for Forest Mycology Research, Northern Research Station, USDA-U.S. Forest Service, One Gifford Pincheot Dr., Madison, WI 53726, USA 5Agricultural Institute of Slovenia, Hacquetova 17, 1000 Ljubljana, Slovenia 6CBS-KNAW Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands 7Institute of Ecology and Earth Sciences and Natural History Museum, University of Tartu, Vanemuise 46, 51014 Tartu, Estonia 8Jodrell Laboratory, Royal Botanic Gardens, Kew, Surrey TW9 3AB, UK 9Sporometrics, Inc., 219 Dufferin Street, Suite 20C, Toronto, Ontario, Canada M6K 1Y9 10Department of Plant Pathology and Environmental Microbiology, 121 Buckhout Laboratory, The Pennsylvania State University, University Park, PA 16802 USA 11State -

Novel Species of Calonectria Associated with Eucalyptus Leaf Blight in Southeast China
Persoonia 26, 2011: 1–12 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE doi:10.3767/003158511X555236 Novel species of Calonectria associated with Eucalyptus leaf blight in Southeast China S.F. Chen1,2, L. Lombard1, J. Roux1, Y.J. Xie2, M.J. Wingfield1, X.D. Zhou1,2 Key words Abstract Leaf blight caused by Calonectria spp. is an important disease occurring on Eucalyptus trees grown in plantations of Southeast Asia. Symptoms of leaf blight caused by Calonectria spp. have recently been observed Cylindrocladium in commercial Eucalyptus plantations in FuJian Province in Southeast China. The aim of this study was to identify Eucalyptus plantations these Calonectria spp. employing morphological characteristics, DNA sequence comparisons for the -tubulin, FuJian β histone H3 and translation elongation factor-1 gene regions and sexual compatibility. Four Calonectria spp. were pathogenicity α identified, including Ca. pauciramosa and three novel taxa described here as Ca. crousiana, Ca. fujianensis and Ca. pseudocolhounii. Inoculation tests showed that all four Calonectria spp. found in this study were pathogenic on two different E. urophylla × E. grandis hybrid clones, commercially utilised in eucalypt plantations in China. Article info Received: 2 July 2010; Accepted: 28 October 2010; Published: 10 January 2011. INTRODUCTION In South and Southeast Asia, CLB is one of the most prominent diseases associated with Eucalyptus trees grown in commercial Species of Calonectria (Ca.) (anamorph state: Cylindrocladium plantations (Old et al. 2003). In these regions, CLB is caused by (Cy.)) are pathogenic to a wide range of plant hosts in tropical several Calonectria spp., including Ca. asiatica, Ca. brassicae, and subtropical areas of the world (Crous & Wingfield 1994, Ca. -

Calonectria Spp. Causing Leaf Spot, Crown and Root Rot of Ornamental Plants in Tunisia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Persoonia 27, 2011: 73–79 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE http://dx.doi.org/10.3767/003158511X615086 Calonectria spp. causing leaf spot, crown and root rot of ornamental plants in Tunisia L. Lombard1, G. Polizzi2*, V. Guarnaccia 2, A. Vitale 2, P.W. Crous1 Key words Abstract Calonectria spp. are important pathogens of ornamental plants in nurseries, especially in the Northern Hemisphere. They are commonly associated with a wide range of disease symptoms of roots, leaves and shoots. Calonectria During a recent survey in Tunisia, a number of Calonectria spp. were isolated from tissues of ornamental plants crown and root rot showing symptoms of leaf spot, crown and root rot. The aim of this study was to identify these Calonectria spp. using DNA phylogeny morphological and DNA sequence comparisons. Two previously undescribed Calonectria spp., C. pseudomexicana leaf spot sp. nov. and C. tunisiana sp. nov., were recognised. Calonectria mexicana and C. polizzii are newly reported for the pathogenicity African continent. Pathogenicity tests with all four Calonectria spp. showed that they are able to cause disease on systematics seedlings of Callistemon spp., Dodonaea viscosa, Metrosideros spp. and Myrtus communis. Article info Received: 5 September 2011; Accepted: 15 October 2011; Published: 18 November 2011. INTRODUCTION MATERIALS AND METHODS Species of Calonectria are common pathogens of a wide Disease survey and isolates range of plant hosts in nurseries cultivated through seedings During November 2010, an ornamental nursery located in or vegetative propagation (Crous 2002, Lombard et al. -

MGS-FD-Boxwood-Blight-.Pdf
7/24/13 Training Outline 1. Introduction, Biology, and Identification 2. Managing Boxwood Blight 3. Other Diseases and Insect Problems on Boxwood 4. Approaches to Diagnosis of Plant Problems Molly Giesbrecht Extension Associate Texas Plant Disease Diagnostic Laboratory History and Current Distribution First discovered in the UK in the mid-1990’s Origin unknown Now spread throughout Europe First found in U.S. in 2011 (CT and NC) U.S. states with confirmed reports: Connecticut, Maryland, Massachusetts, New York, North Carolina, Ohio, Oregon, Pennsylvania, Rhode Island, and Virginia Also present in New Zealand and Canada Distribution of boxwood blight in US Regulations Not federally regulated by the USDA Some states have put regulations in place to try to limit disease spread Federal research money focused on preventing introduction to new areas and managing the disease once established 1 7/24/13 Research Efforts Disease Triangle USDA Farm Bill Research funding for: A susceptible host Development of rapid diagnostics Studying fungal epidemiology Fungicide trials Studying effective cultural practices USDA Agricultural Research Initiative funding for: Breeding for boxwood blight resistance A capable An environment pathogen conducive to disease Figure credit: Ed Zaborski, University of Illinois FUNGI AND OOMYCETES FUNGI AND OOMYCETES Characteristics and spread: Characteristics and spread cont’d: ¨ Grow vegetatively by hyphae (tubular filaments) Reproduce via sexual and asexual reproduction to ¤ Hyphae grow radially to spread within a plant and sometimes produce spores from plant to plant through root contacts or in soil often produced in/on specialized structures, some of which are big enough to see, i.e. mushrooms Dispersed by wind, animal, rain splash, soil water, equipment, or other means FUNGI AND OOMYCETES Pathogen vs. -

Investigating Soilborne Nectriaceous Fungi Impacting Avocado Tree
Investigating soilborne nectriaceous fungi impacting avocado tree establishment in Australia Louisamarie Elicano Parkinson Bachelor of Biotechnology (Honours Class 1) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2017 Queensland Alliance for Agriculture and Food Innovation 1 Abstract Black root rot is a severe disease of nursery avocado trees and orchard transplants caused by soilborne fungal pathogens in the Nectriaceae family. The genera reported to be associated with black root rot are Calonectria, Cylindrocladiella, Dactylonectria, Gliocladiopsis and Ilyonectria. These genera have not been widely studied in avocado, although the disease causes significant commercial loss, with symptoms including black, rotten roots; tree stunting; leaf wilt; and rapid tree decline and death. This PhD research aims to i) identify the nectriaceous fungal species found in avocado roots in Australia, using morphological studies and molecular phylogenetic analyses of fungal gene sequences; ii) to perform pathogenicity tests on avocado seedlings and fruit to determine the pathogenic species; iii) to investigate whether the pathogens produce phytotoxic exudates which induce and facilitate disease symptom development; iv) and to use the generated gene sequence data to develop a molecular diagnostic for rapidly detecting the pathogens. Fungal isolates were obtained from symptomatic roots from sick and healthy avocado trees, nursery stock, young orchard transplants and mature established orchard trees from all growing regions in Australia, and from other host species. Bayesian inference and Maximum likelihood phylogenetic analyses of concatenated ITS, β-tubulin and histone H3 gene loci were used to identify and classify 153 Nectriaceae isolates in the genera Calonectria, Cylindrocladiella, Dactylonectria, Gliocladiopsis, Ilyonectria and Mariannaea. -

Novel Species of Gliocladiopsis (Nectriaceae, Hypocreales, Ascomycota) from Avocado Roots (Persea Americana) in Australia
mycoscience 58 (2017) 95e102 Available online at www.sciencedirect.com journal homepage: www.elsevier.com/locate/myc Full paper Novel species of Gliocladiopsis (Nectriaceae, Hypocreales, Ascomycota) from avocado roots (Persea americana) in Australia * Louisamarie E. Parkinson a, Roger G. Shivas b, Elizabeth K. Dann a, a Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Ecosciences Precinct, 41 Boggo Road, Dutton Park, QLD 4102, Australia b Plant Pathology Herbarium, Department of Agriculture and Fisheries, Ecosciences Precinct, 41 Boggo Road, Dutton Park, QLD 4102, Australia article info abstract Article history: Root rot of avocado (Persea americana) is an important disease in seedling nurseries as well Received 24 June 2016 as in the field in eastern and southern Australia. During an investigation into the causal Received in revised form organisms of avocado root rot, 19 isolates of Gliocladiopsis were obtained from necrotic 27 October 2016 lesions on avocado roots and examined by morphology and comparison of DNA sequences Accepted 30 October 2016 from three gene loci (the internal transcribed spacer region of the nuclear rDNA, Histone Available online 7 December 2016 H3 and b-tubulin). Three new species of Gliocladiopsis are described as a result of phylo- genetic analysis of these data. One of the new species, G. peggii, formed a monophyletic Keywords: group that may represent an unresolved species complex as it contained a polytomy that Lauraceae included a well-supported clade comprising two subclades. Gliocladiopsis peggii is sister to Phylogeny G. mexicana, which is known from soil in Mexico. The remaining two new species, G. whileyi Rhizosphere and G. -

University of Catania
UNIVERSITY OF CATANIA DEPARTMENT OF AGROFOOD AND ENVIRONMENTAL MANAGEMENT SYSTEMS INTERNATIONAL PhD PLANT HEALTH TECHNOLOGIES AND PROTECTION OF AGROECOSYSTEMS CYCLE XXV 2010-2012 Detection of new Calonectria spp. and Calonectria Diseases and Changes in Fungicide Sensitivity in Calonectria scoparia Complex This thesis is presented for the degree of Doctor of Philosophy by VLADIMIRO GUARNACCIA COORDINATOR SUPERVISOR PROF. C. RAPISARDA PROF. G.POLIZZI CHAPTER 1 - The genus Calonectria and the fungicide resistance.......................... 1 1.1 Introduction............................................................................................................ 2 1.1.1 Calonectria...................................................................................................... 2 1.1.2 Importance of Calonectria.............................................................................. 3 1.1.3 Morphology..................................................................................................... 6 1.1.4 Pathogenicity................................................................................................... 9 1.1.5 Microsclerotia ................................................................................................. 9 1.1.6 Mating compatibility..................................................................................... 10 1.1.7 Phylogeny...................................................................................................... 12 1.1.7.1 Calonectria scoparia species complex .................................................