Investigating Soilborne Nectriaceous Fungi Impacting Avocado Tree
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calonectria Ilicicola
OctoberPathogen of11 the month –October 2011 a b c d Reitsma & Fig. 1. Calonectria ilicicola; asci with ascospores (a); microsclerotia on a papaya root (b); conidium of anamorph, Cylindrocladium parasiticum (c); orange perithecia on papaya root (d); Photo credits M. Male (a,b,c), L. Vawdrey (d) Disease: Collar rot of papaya Pathogen: Calonectria ilicicola (anamorph: Cylindrocladium parasiticum) Boedijn Classification: K: Fungi, D: Ascomycota, C: Sordariomycetes, O: Hypocreales, F: Nectriaceae Calonectria ilicicola (Fig. 1) is a fungal pathogen found throughout the world infecting a wide variety of crops. It is best known as the causal agent of peg, pod and root necrosis of peanuts, collar rot of koa, leaf spot in some eucalypts and various root and collar rots of soybean, anthurium, groundnut and lucerne. In Australia, it causes black rot of peanut and collar rot of papaya. Its development is favoured by poorly drained soils. Host Range: Key Distinguishing Features: C. ilicicola is found throughout the world. It is In seedlings, the initial symptoms are of a discoloured pathogenic on a wide variety of plants including water soaked, root collar. As this rot develops, plants peanuts, soybean, anthurium, eucalypts, lucerne, are stunted and leaves become chlorotic and wilt. groundnut and papaya. Eventually, orange to red perithecia and thick-walled ilicicola brown microsclerotia will form on roots at or near the Impact: soil line (Fig 1 b,d). In culture, asci are clavate, 90- In far north Queensland, collar rot of papaya caused 140 x 12-19μm, tapering to a long thin stalk by C. ilicicola affects young plants in poorly drained containing eight hyaline ascospores (Fig1,a). -

IDENTIFICAÇÃO DE ESPÉCIES DE Calonectria E REAÇÃO DE ACESSOS DE GRÃO-DE-BICO a ISOLADOS DE Calonectria Brassicae
UNIVERSIDADE DE BRASÍLIA INSTITUTO DE CIÊNCIAS BIOLÓGICAS DEPARTAMENTO DE FITOPATOLOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM FITOPATOLOGIA IDENTIFICAÇÃO DE ESPÉCIES DE Calonectria E REAÇÃO DE ACESSOS DE GRÃO-DE-BICO A ISOLADOS DE Calonectria brassicae NARA LÚCIA SOUZA RIBEIRO TRINDADE Brasília – DF 2019 NARA LÚCIA SOUZA RIBEIRO TRINDADE IDENTIFICAÇÃO DE ESPÉCIES DE Calonectria E REAÇÃO DE ACESSOS DE GRÃO-DE-BICO A ISOLADOS DE Calonectria brassicae Dissertação apresentada à Universidade de Brasília como requisito parcial para obtenção do título de Mestre em Fitopatologia pelo Programa de Pós- Graduação em Fitopatologia. Orientador Luiz Eduardo Bassay Blum, Doutor em Fitopatologia BRASÍLIA DISTRITO FEDERAL – BRASIL 2019 FICHA CATALOGRÁFICA Trindade, Nara Lúcia Souza Ribeiro. Identificação de espécies de Calonectria e reação de acessos de grão-de-bico a isolados de Calonectria brassicae / Nara Lúcia Souza Ribeiro Trindade. Brasília, 2019. 60 p.: il. Dissertação de mestrado. Programa de Pós-Graduação em Fitopatologia, Universidade de Brasília, Brasília. 1. Grão-de-bico – CalonectriaI. I. Universidade de Brasília. PPG/FIT. II. Identificação de espécies de Calonectria spp. e reação de acessos de grão-de-bico a isolados de Calonectria brassicae. Trabalho realizado junto ao Departamento de Fitopatologia do Instituto de Ciências Biológicas da Universidade de Brasília, sob Orientação do Professor Dr. Luiz Eduardo Bassay Blum, com apoio da Empresa Brasileira de Pesquisa Agropecuária. IDENTIFICAÇÃO DE ESPÉCIES DE Calonectria E REAÇÃO DE ACESSOS DE GRÃO-DE-BICO A ISOLADOS DE Calonectria brassicae NARA LÚCIA SOUZA RIBEIRO TRINDADE DISSERTAÇÃO APROVADA em: 17/12/2019 por: __________________________________ Dra. Cléia Santos Cabral UNIDESC (Examinador Externo) __________________________________ Dr. Leonardo Silva Boiteux Embrapa Hortaliças (Examinador Externo – Vinculado ao PPG-FIT) __________________________________ Dr. -

Cylindrocladium Buxicola Nom. Cons. Prop.(Syn. Calonectria
I Promotors: Prof. dr. ir. Monica Höfte Laboratory of Phytopathology, Department of Crop Protection Faculty of Bioscience Engineering Ghent University Dr. ir. Kurt Heungens Institute for Agricultural and Fisheries Research (ILVO) Plant Sciences Unit - Crop Protection Dean: Prof. dr. ir. Guido Van Huylenbroeck Rector: Prof. dr. Anne De Paepe II Bjorn Gehesquière Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences III Dutch translation of the title: Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) in Buxus: moleculaire karakterisering, epidemiologie, waardplantresistentie en chemische bestrijding. Please refer to this work as follows: Gehesquière B. (2014). Cylindrocladium buxicola nom. cons. prop. (syn. Calonectria pseudonaviculata) on Buxus: molecular characterization, epidemiology, host resistance and fungicide control. Phd Thesis. Ghent University, Belgium The author and the promotors give authorisation to consult and to copy parts of this work for personal use only. Any other use is limited by Laws of Copyright. Permission to reproduce any material contained in this work should be obtained from the author. The promotors, The author, Prof. dr. ir. M. Höfte Dr. ir. K. Heungens ir. B. Gehesquière IV Een woordje van dank…. Dit dankwoord schrijven is ongetwijfeld het leukste onderdeel van deze thesis, en een mooie afsluiting van een interessante periode. Terugblikkend op de voorbije vier jaren kan ik enkel maar beamen dat een doctoraat zoveel meer is dan een wetenschappelijke uitdaging. Het is een levensreis in al zijn facetten, waarbij ik mezelf heb leren kennen in al mijn goede en slechte kantjes. -

Phylogeny and Systematics of the Genus Calonectria
available online at www.studiesinmycology.org Studies in Mycology 66: 31–69. 2010. doi:10.3114/sim.2010.66.03 Phylogeny and systematics of the genus Calonectria L. Lombard1*, P.W. Crous2, B.D. Wingfield3 and M.J. Wingfield1 1Department of Microbiology and Plant Pathology, Tree Protection Co-operative Programme, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria 0002, South Africa; 2CBS-KNAW Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands; 3Department of Genetics, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria 0002, South Africa *Correspondence: Lorenzo Lombard, [email protected] Abstract: Species of Calonectria are important plant pathogens, several of which have a worldwide distribution. Contemporary taxonomic studies on these fungi have chiefly relied on DNA sequence comparisons of the β-tubulin gene region. Despite many new species being described, there has been no phylogenetic synthesis for the group since the last monographic study almost a decade ago. In the present study, the identity of a large collection of Calonectria isolates from various geographic regions was determined using morphological and DNA sequence comparisons. This resulted in the discovery of seven new species; Ca. densa, Ca. eucalypti, Ca. humicola, Ca. orientalis, Ca. pini, Ca. pseudoscoparia and Ca. sulawesiensis, bringing the total number of currently accepted Calonectria species to 68. A multigene phylogeny was subsequently constructed for all available Calonectria spp., employing seven gene regions, namely actin, β-tubulin, calmodulin, histone H3, the internal transcribed spacer regions 1 and 2 and the 5.8S gene of the ribosomal RNA, 28S large subunit RNA gene and translation elongation 1-alpha. -

Epidemiological Studies on the Infection Process and Symptom Expression of Soybean Sudden Death Syndrome Carlos Cecilio Gongora-Canul Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2010 Epidemiological studies on the infection process and symptom expression of soybean sudden death syndrome Carlos Cecilio Gongora-canul Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Plant Pathology Commons Recommended Citation Gongora-canul, Carlos Cecilio, "Epidemiological studies on the infection process and symptom expression of soybean sudden death syndrome" (2010). Graduate Theses and Dissertations. 11510. https://lib.dr.iastate.edu/etd/11510 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Epidemiological studies on the infection process and symptom expression of soybean sudden death syndrome by Carlos Cecilio Góngora-Canul A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Pathology Program of Study Committee: Leonor Leandro, Major Professor Gary Munkvold Greg Tylka X. B Yang Dan Nordman Iowa State University Ames, Iowa 2010 Copyright © Carlos Cecilio Góngora-Canul, 2010. All rights reserved. ii DEDICATION To my Lord, for giving me the blessing and the adventure to live. To my mother Elsa and my father Elias for their endless love and to all my brothers and sisters (Javier, Roberto, Martha, Manuel, Enrique and Nicte-Há) for all their great love affection. -

Pathogenicity and Molecular Detection of Nectriaceous Fungi Associated with Black Root Rot of Avocado
IX World Avocado Congress, 23 – 27 September, 2019, Medellín, Colombia WAC-130 PATHOGENICITY AND MOLECULAR DETECTION OF NECTRIACEOUS FUNGI ASSOCIATED WITH BLACK ROOT ROT OF AVOCADO L. E. Parkinson, D. P. Le, R. G. Shivas and E. K. Dann Dr Louisamarie Parkinson BBiotech(Hons), PhD Centre for Horticultural Science Queensland Alliance for Agriculture and Food Innovation (QAAFI) The University of Queensland, Brisbane Qld 4072 Australia [email protected] | https://www.qaafi.uq.edu.au THE UNIVERSITY OF QUEENSLAND St Lucia, Brisbane, Queensland Australia PATHOGENICITY AND MOLECULAR DETECTION OF NECTRIACEOUS FUNGI ASSOCIATED WITH BLACK ROOT ROT OF AVOCADO L. E. Parkinson1, D. P. Le1, R. G. Shivas2, E. K. Dann1 1 Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Australia 2Centre for Crop Health, The University of Southern Queensland, Australia KEY WORDS Calonectria, Calonectria ilicicola, Dactylonectria, Dactylonectria macrodidyma, diagnostic test, diversity, loop-mediated isothermal amplification (LAMP) SUMMARY Black root rot of avocado associated with soilborne nectriaceous fungi is an aggressive disease of nursery trees and young orchards transplants, causing tree stunting, wilt, severe root necrosis, rapid decline and death within a year after planting. This study aimed to identify the fungal genera associated with the disease, determine the causal agents of black root rot, and develop a rapid molecular test for detection of key pathogens in avocado roots. A disease survey in all Australian growing regions collected 153 nectriaceous fungal isolates from roots of 91 symptomatic and healthy avocado trees and other hosts including peanut, papaya, blueberry, custard apple and grapevine. The fungal isolates were identified with phylogenetic analyses of ITS, β-tubulin and Histone H3 sequenced genes. -

Novel Species of Cylindrocarpon (Neonectria) and Campylocarpon Gen
STUDIES IN MYCOLOGY 50: 431–455. 2004. Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.) Francois Halleen1, Hans-Josef Schroers2,3*, Johannes Z. Groenewald3 and Pedro W. Crous3 1ARC Infruitec-Nietvoorbij (The Fruit, Vine and Wine Institute of the Agricultural Research Council), P. Bag X5026, Stellen- bosch, 7599, and the Department of Plant Pathology, University of Stellenbosch, P. Bag X1, Matieland 7602, South Africa; 2Agricultural Institute of Slovenia, Hacquetova 17, p.p. 2553, 1001 Ljubljana, Slovenia; 3Centraalbureau voor Schimmelcul- tures, P.O. Box 85167, NL-3508 AD Utrecht, The Netherlands *Correspondence: Hans-Josef Schroers, [email protected] Abstract: Four Cylindrocarpon or Cylindrocarpon-like taxa isolated from asymptomatic or diseased Vitis vinifera plants in nurseries and vineyards of South Africa, New Zealand, Australia, and France were morphologically and phylogenetically compared with other Neonectria/Cylindrocarpon taxa. Sequences of the partial nuclear large subunit ribosomal DNA (LSU rDNA), internal transcribed spacers 1 and 2 of the rDNA including the 5.8S rDNA gene (ITS), and partial ȕ-tubulin gene introns and exons were used for phylogenetic inference. Neonectria/Cylindrocarpon species clustered in mainly three groups. One monophyletic group consisted of three subclades comprising (i) members of the Neonectria radicicola/Cylindrocarpon destructans complex, which contained strains isolated from grapevines in South Africa, New Zealand, and France; (ii) a Neonectria/Cylindrocarpon species isolated from grapevines in South Africa, Canada (Ontario), Australia (Tasmania), and New Zealand, described here as Cylindrocarpon macrodidymum; and (iii) an assemblage of species closely related to strains identified as Cylindrocarpon cylindroides, the type species of Cylindrocarpon. -
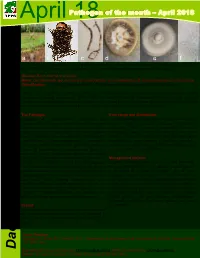
Dactylonectria Lombard & Crous
April Pathogen18 of the month – April 2018 a b c d e f Fig. 1. Black root rot symptoms in young orchard transplants (a), necrotic avocado roots (b, c) Dactylonectria macrodidyma on ½ sPDA at 3.75 cm after 10 days growth (d, e), D. macrodidyma macroconidia at 40 × magnification (f) Disease: Black root rot of avocado Name: Dactylonectria spp. including D. macrodidyma, D. novozelandica, D. pauciseptata and D. anthuriicola Classification: K: Fungi, D: Ascomycota, C: Sordariomycetes, O: Hypocreales, F: Nectriaceae Black root rot caused by nectriaceous fungi is a severe disease of avocado nursery trees and young orchard transplants, causing decline and death within one year of planting. Symptoms include stunting, wilt, leaf chlorosis and browning, leaf drop prior to tree death caused by severe necrosis of the root system. In Australia black root rot of avocado is caused by Calonectria ilicicola and several Dactylonectria spp. The Pathogen: Host range and distribution: Species of Dactylonectria (reported as Dactylonectria spp. cause root rot diseases in various Cylindrocarpon in older literature) have often been hosts including avocado (Persea americana), isolated from necrotic avocado roots. Dactylonectria grapevine (Vitis vinifera), cherimoya (Annona macrodidyma is the most prevalent of the pathogens cherimola), kiwifruit (Actinidia deliciosa) and olive found in symptomatic avocado roots. Dactylonectria (Olea europaea). Dactylonectria spp. associated with novozelandica, D. pauciseptata and D. anthuriicola avocado have been reported in Australia and Italy. have also been isolated from avocado roots and However the fungal genus is reported globally across Lombard & Crous shown to be pathogenic in glasshouse tests with numerous horticultural industries. seedlings. While Dactylonectria spp. -

Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and Related Genera with Cylindrocarpon-Like Anamorphs
available online at www.studiesinmycology.org StudieS in Mycology 68: 57–78. 2011. doi:10.3114/sim.2011.68.03 Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs P. Chaverri1*, C. Salgado1, Y. Hirooka1, 2, A.Y. Rossman2 and G.J. Samuels2 1University of Maryland, Department of Plant Sciences and Landscape Architecture, 2112 Plant Sciences Building, College Park, Maryland 20742, USA; 2United States Department of Agriculture, Agriculture Research Service, Systematic Mycology and Microbiology Laboratory, Rm. 240, B-010A, 10300 Beltsville Avenue, Beltsville, Maryland 20705, USA *Correspondence: Priscila Chaverri, [email protected] Abstract: Neonectria is a cosmopolitan genus and it is, in part, defined by its link to the anamorph genusCylindrocarpon . Neonectria has been divided into informal groups on the basis of combined morphology of anamorph and teleomorph. Previously, Cylindrocarpon was divided into four groups defined by presence or absence of microconidia and chlamydospores. Molecular phylogenetic analyses have indicated that Neonectria sensu stricto and Cylindrocarpon sensu stricto are phylogenetically congeneric. In addition, morphological and molecular data accumulated over several years have indicated that Neonectria sensu lato and Cylindrocarpon sensu lato do not form a monophyletic group and that the respective informal groups may represent distinct genera. In the present work, a multilocus analysis (act, ITS, LSU, rpb1, tef1, tub) was applied to representatives of the informal groups to determine their level of phylogenetic support as a first step towards taxonomic revision of Neonectria sensu lato. Results show five distinct highly supported clades that correspond to some extent with the informal Neonectria and Cylindrocarpon groups that are here recognised as genera: (1) N. -

Novel Species of Cylindrocarpon (Neonectria) and Campylocarpon Gen
STUDIES IN MYCOLOGY 50: 431–455. 2004. Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.) Francois Halleen1, Hans-Josef Schroers2,3*, Johannes Z. Groenewald3 and Pedro W. Crous3 1ARC Infruitec-Nietvoorbij (The Fruit, Vine and Wine Institute of the Agricultural Research Council), P. Bag X5026, Stellen- bosch, 7599, and the Department of Plant Pathology, University of Stellenbosch, P. Bag X1, Matieland 7602, South Africa; 2Agricultural Institute of Slovenia, Hacquetova 17, p.p. 2553, 1001 Ljubljana, Slovenia; 3Centraalbureau voor Schimmelcul- tures, P.O. Box 85167, NL-3508 AD Utrecht, The Netherlands *Correspondence: Hans-Josef Schroers, [email protected] Abstract: Four Cylindrocarpon or Cylindrocarpon-like taxa isolated from asymptomatic or diseased Vitis vinifera plants in nurseries and vineyards of South Africa, New Zealand, Australia, and France were morphologically and phylogenetically compared with other Neonectria/Cylindrocarpon taxa. Sequences of the partial nuclear large subunit ribosomal DNA (LSU rDNA), internal transcribed spacers 1 and 2 of the rDNA including the 5.8S rDNA gene (ITS), and partial -tubulin gene introns and exons were used for phylogenetic inference. Neonectria/Cylindrocarpon species clustered in mainly three groups. One monophyletic group consisted of three subclades comprising (i) members of the Neonectria radicicola/Cylindrocarpon destructans complex, which contained strains isolated from grapevines in South Africa, New Zealand, and France; (ii) a Neonectria/Cylindrocarpon species isolated from grapevines in South Africa, Canada (Ontario), Australia (Tasmania), and New Zealand, described here as Cylindrocarpon macrodidymum; and (iii) an assemblage of species closely related to strains identified as Cylindrocarpon cylindroides, the type species of Cylindrocarpon. -

Characterisation of Cylindrocarpon Spp
CHARACTERISATION OF CYLINDROCARPON SPP. ASSOCIATED WITH BLACK FOOT DISEASE OF GRAPEVINE Francois Halleen Dissertation presented for the degree of Doctor of Philosophy in Agriculture at the University of Stellenbosch Promoter: Prof. Pedro W. Crous Co-promoter: Dr. Paul H. Fourie December 2005 DECLARATION I, the undersigned, hereby declare that the work contained in this dissertation is my own original work and that I have not previously in its entirety or in part been submitted it at any university for a degree. Signature: Date: http://scholar.sun.ac.za CHARACTERISATION OF CYLINDROCARPON SPP. ASSOCIATED WITH BLACK FOOT DISEASE OF GRAPEVINE SUMMARY During the past few years a drastic reduction has been noted in the survival rate of grafted grapevines in nurseries, as well as in young vineyards in the Western Cape Province of South Africa. Circumstantial evidence suggested that Cylindrocarpon spp., which cause black foot disease of grapevine, were associated with this decline. Black foot disease of grapevine is a relatively new, and as yet poorly known disease affecting vines in various countries where grapevines are cultivated. Primary aims of this research have been (1) to conduct nursery surveys in order to determine which fungi are involved in the decline phenomenon, with special reference to the involvement of Cylindrocarpon spp., (2) to identify and characterise the organisms believed to be the causal organisms of black foot disease, and (3) the development of control and/or management strategies to prevent or eradicate Cylindrocarpon infections. Nursery grapevines were sampled at different stages from three commercial nurseries in the Wellington area of the Western Cape Province and were investigated during the 19992000 season by means of destructive sampling. -

Dactylonectria and Ilyonectria Species Causing Black Foot Disease of Andean Blackberry (Rubus Glaucus Benth) in Ecuador
diversity Article Dactylonectria and Ilyonectria Species Causing Black Foot Disease of Andean Blackberry (Rubus Glaucus Benth) in Ecuador Jessica Sánchez 1, Paola Iturralde 1, Alma Koch 1, Cristina Tello 2, Dennis Martinez 1, Natasha Proaño 1, Anibal Martínez 3, William Viera 3 , Ligia Ayala 1 and Francisco Flores 1,4,* 1 Departamento de Ciencias de la Vida y la Agricultura, Universidad de las Fuerzas Armadas-ESPE, Av. General Rumiñahui, Sangolquí 171103, Ecuador; [email protected] (J.S.); [email protected] (P.I.); [email protected] (A.K.); [email protected] (D.M.); [email protected] (N.P.); [email protected] (L.A.) 2 Plant Protection Department, National Institute of Agricultural Research (INIAP), Panamericana Sur km 1, Cutuglahua 171108, Ecuador; [email protected] 3 Fruit Program, National Institute of Agricultural Research (INIAP), Av. Interoceánica km 14 1⁄2, Tumbaco 170184, Ecuador; [email protected] (A.M.); [email protected] (W.V.) 4 Centro de Investigación de Alimentos, CIAL, Facultad de Ciencias de la Ingeniería e Industrias, Universidad UTE, Quito 170147, Ecuador * Correspondence: fjfl[email protected] Received: 7 October 2019; Accepted: 29 October 2019; Published: 14 November 2019 Abstract: Andean blackberry (Rubus glaucus Benth) plants from the provinces of Tungurahua and Bolivar (Ecuador) started showing symptoms of black foot disease since 2010. Wilted plants were sampled in both provinces from 2014 to 2017, and fungal isolates were obtained from tissues surrounding necrotic lesions in the cortex of the roots and crown. Based on morphological characteristics and DNA sequencing of histone 3 and the translation elongation factor 1α gene, isolates were identified as one of seven species, Ilyonectria vredehoekensis, Ilyonectria robusta, Ilyonectria venezuelensis, Ilyonectria europaea, Dactylonectria torresensis, or Dactylonectria novozelandica.