Predicting Stroke Severity with a 3-Min Recording from the Muse Portable
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Brief March 2017 Publication #2017-16
Research Brief March 2017 Publication #2017-16 Flourishing From the Start: What Is It and How Can It Be Measured? Kristin Anderson Moore, PhD, Child Trends Christina D. Bethell, PhD, The Child and Adolescent Health Measurement Introduction Initiative, Johns Hopkins Bloomberg School of Every parent wants their child to flourish, and every community wants its Public Health children to thrive. It is not sufficient for children to avoid negative outcomes. Rather, from their earliest years, we should foster positive outcomes for David Murphey, PhD, children. Substantial evidence indicates that early investments to foster positive child development can reap large and lasting gains.1 But in order to Child Trends implement and sustain policies and programs that help children flourish, we need to accurately define, measure, and then monitor, “flourishing.”a Miranda Carver Martin, BA, Child Trends By comparing the available child development research literature with the data currently being collected by health researchers and other practitioners, Martha Beltz, BA, we have identified important gaps in our definition of flourishing.2 In formerly of Child Trends particular, the field lacks a set of brief, robust, and culturally sensitive measures of “thriving” constructs critical for young children.3 This is also true for measures of the promotive and protective factors that contribute to thriving. Even when measures do exist, there are serious concerns regarding their validity and utility. We instead recommend these high-priority measures of flourishing -

The Origin of the Peculiarities of the Vietnamese Alphabet André-Georges Haudricourt
The origin of the peculiarities of the Vietnamese alphabet André-Georges Haudricourt To cite this version: André-Georges Haudricourt. The origin of the peculiarities of the Vietnamese alphabet. Mon-Khmer Studies, 2010, 39, pp.89-104. halshs-00918824v2 HAL Id: halshs-00918824 https://halshs.archives-ouvertes.fr/halshs-00918824v2 Submitted on 17 Dec 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published in Mon-Khmer Studies 39. 89–104 (2010). The origin of the peculiarities of the Vietnamese alphabet by André-Georges Haudricourt Translated by Alexis Michaud, LACITO-CNRS, France Originally published as: L’origine des particularités de l’alphabet vietnamien, Dân Việt Nam 3:61-68, 1949. Translator’s foreword André-Georges Haudricourt’s contribution to Southeast Asian studies is internationally acknowledged, witness the Haudricourt Festschrift (Suriya, Thomas and Suwilai 1985). However, many of Haudricourt’s works are not yet available to the English-reading public. A volume of the most important papers by André-Georges Haudricourt, translated by an international team of specialists, is currently in preparation. Its aim is to share with the English- speaking academic community Haudricourt’s seminal publications, many of which address issues in Southeast Asian languages, linguistics and social anthropology. -
Applications the Formula Y = Mx + B Sometimes Appears with Different
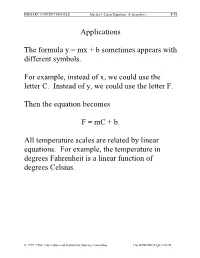
PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-71 Applications The formula y = mx + b sometimes appears with different symbols. For example, instead of x, we could use the letter C. Instead of y, we could use the letter F. Then the equation becomes F = mC + b. All temperature scales are related by linear equations. For example, the temperature in degrees Fahrenheit is a linear function of degrees Celsius. © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-72 Basic Temperature Facts Water freezes at: 0°C, 32°F Water Boils at: 100°C, 212°F F • (100, 212) • (0, 32) C Can you solve for m and b in F = mC + b? © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-73 To find the equation relating Fahrenheit to Celsius we need m and b F – F m = 2 1 C2 – C1 212 – 32 m = 100 – 0 180 9 m = = 100 5 9 Therefore F = C + b 5 To find b, substitute the coordinates of either point. 9 32 = (0) + b 5 Therefore b = 32 Therefore the equation is 9 F = C + 32 5 Can you solve for C in terms of F? © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-74 Celsius in terms of Fahrenheit 9 F = C + 32 5 5 5 9 F = æ C +32ö 9 9è 5 ø 5 5 F = C + (32) 9 9 5 5 F – (32) = C 9 9 5 5 C = F – (32) 9 9 5 C = (F – 32) 9 Example: How many degrees Celsius is77°F? 5 C = (77 – 32) 9 5 C = (45) 9 C = 25° So 72°F = 25°C © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-75 Standard 8 Algebra I, Grade 8 Standards Students understand the concept of parallel and perpendicular lines and how their slopes are related. -

Form Y-203-I, If You Had More Than One Job, Or If You Had a Job for Only Part of the Year
Department of Taxation and Finance Yonkers Nonresident Earnings Tax Return Y-203 For the full year January 1, 2020, through December 31, 2020, or fiscal year beginning and ending Name as shown on Form IT-201 or IT-203 Social Security number A Were you a Yonkers resident for any part of the taxable year? (mark an X in the appropriate box) Yes No (see instructions) (See the instructions for Form IT-201 or IT-203 for the definition of a resident.) If Yes: 1. Give period of Yonkers residence. From (mmddyyyy) to (mmddyyyy) 2. Are you reporting Yonkers resident income tax surcharge on your New York State return? ..................................................................................... Yes No (submit explanation) 3. You must complete and submit Form IT-360.1 (see instructions). B Did you or your spouse maintain an apartment or other living quarters in Yonkers during any part of the year?...................................................................................... Yes No If Yes, give address below and enter the number of days spent in Yonkers during 2020: days Address: C Are you reporting income from self-employment (on line 2 below)?......... Yes No If Yes, complete the following: Business name Business address Employer identification number Principal business activity Form of business: Sole proprietorship Partnership Other (explain) Calculation of nonresident earnings tax 1 Gross wages and other employee compensation (see instructions; if claiming an allocation, include amount from line 22) ............................................... 1 .00 2 Net earnings from self-employment (see instructions; if claiming an allocation, include amount from line 32; if a loss, write loss on line 2) ............................................................................................... 2 .00 3 Add lines 1 and 2 (if line 2 is a loss, enter amount from line 1) ........................................................... -

Ligature Modeling for Recognition of Characters Written in 3D Space Dae Hwan Kim, Jin Hyung Kim
Ligature Modeling for Recognition of Characters Written in 3D Space Dae Hwan Kim, Jin Hyung Kim To cite this version: Dae Hwan Kim, Jin Hyung Kim. Ligature Modeling for Recognition of Characters Written in 3D Space. Tenth International Workshop on Frontiers in Handwriting Recognition, Université de Rennes 1, Oct 2006, La Baule (France). inria-00105116 HAL Id: inria-00105116 https://hal.inria.fr/inria-00105116 Submitted on 10 Oct 2006 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Ligature Modeling for Recognition of Characters Written in 3D Space Dae Hwan Kim Jin Hyung Kim Artificial Intelligence and Artificial Intelligence and Pattern Recognition Lab. Pattern Recognition Lab. KAIST, Daejeon, KAIST, Daejeon, South Korea South Korea [email protected] [email protected] Abstract defined shape of character while it showed high recognition performance. Moreover when a user writes In this work, we propose a 3D space handwriting multiple stroke character such as ‘4’, the user has to recognition system by combining 2D space handwriting write a new shape which is predefined in a uni-stroke models and 3D space ligature models based on that the and which he/she has never seen. -

ISO Basic Latin Alphabet
ISO basic Latin alphabet The ISO basic Latin alphabet is a Latin-script alphabet and consists of two sets of 26 letters, codified in[1] various national and international standards and used widely in international communication. The two sets contain the following 26 letters each:[1][2] ISO basic Latin alphabet Uppercase Latin A B C D E F G H I J K L M N O P Q R S T U V W X Y Z alphabet Lowercase Latin a b c d e f g h i j k l m n o p q r s t u v w x y z alphabet Contents History Terminology Name for Unicode block that contains all letters Names for the two subsets Names for the letters Timeline for encoding standards Timeline for widely used computer codes supporting the alphabet Representation Usage Alphabets containing the same set of letters Column numbering See also References History By the 1960s it became apparent to thecomputer and telecommunications industries in the First World that a non-proprietary method of encoding characters was needed. The International Organization for Standardization (ISO) encapsulated the Latin script in their (ISO/IEC 646) 7-bit character-encoding standard. To achieve widespread acceptance, this encapsulation was based on popular usage. The standard was based on the already published American Standard Code for Information Interchange, better known as ASCII, which included in the character set the 26 × 2 letters of the English alphabet. Later standards issued by the ISO, for example ISO/IEC 8859 (8-bit character encoding) and ISO/IEC 10646 (Unicode Latin), have continued to define the 26 × 2 letters of the English alphabet as the basic Latin script with extensions to handle other letters in other languages.[1] Terminology Name for Unicode block that contains all letters The Unicode block that contains the alphabet is called "C0 Controls and Basic Latin". -

Form W-4, Employee's Withholding Certificate
Employee’s Withholding Certificate OMB No. 1545-0074 Form W-4 ▶ (Rev. December 2020) Complete Form W-4 so that your employer can withhold the correct federal income tax from your pay. ▶ Department of the Treasury Give Form W-4 to your employer. 2021 Internal Revenue Service ▶ Your withholding is subject to review by the IRS. Step 1: (a) First name and middle initial Last name (b) Social security number Enter Address ▶ Does your name match the Personal name on your social security card? If not, to ensure you get Information City or town, state, and ZIP code credit for your earnings, contact SSA at 800-772-1213 or go to www.ssa.gov. (c) Single or Married filing separately Married filing jointly or Qualifying widow(er) Head of household (Check only if you’re unmarried and pay more than half the costs of keeping up a home for yourself and a qualifying individual.) Complete Steps 2–4 ONLY if they apply to you; otherwise, skip to Step 5. See page 2 for more information on each step, who can claim exemption from withholding, when to use the estimator at www.irs.gov/W4App, and privacy. Step 2: Complete this step if you (1) hold more than one job at a time, or (2) are married filing jointly and your spouse Multiple Jobs also works. The correct amount of withholding depends on income earned from all of these jobs. or Spouse Do only one of the following. Works (a) Use the estimator at www.irs.gov/W4App for most accurate withholding for this step (and Steps 3–4); or (b) Use the Multiple Jobs Worksheet on page 3 and enter the result in Step 4(c) below for roughly accurate withholding; or (c) If there are only two jobs total, you may check this box. -

ENIGMA X Aka SPARKLE Enclosure Manual Ver 7 012017
ENIGMA-X / SPARKLE* ENCLOSURE SHOWER ENCLOSURE INSTALLATION INSTRUCTIONS IMPORTANT DreamLine® reserves the right to alter, modify or redesign products at any time without prior notice. For the latest up-to-date technical drawings, manuals, warranty information or additional details please refer to your model’s web page on DreamLine.com MODEL #s MODEL #s SHEN-6134480-## SHEN-6134720-## SHEN-6134600-## ##=finish *The SPARKLE model name designates an option with MirrorMax patterned glass. 07- Brushed Stainless Steel The installation is identical to the Enigma-X. 08- Polished Stainless Steel Right Hand Return panel installation shown 18- Tuxedo For more information about DreamLine® Shower Doors & Tub Doors please visit DreamLine.com ENIGMA-X / SPARKLE Enclosure manual Ver 7 01/2017 This model is treated with DreamLine’s exclusive ClearMaxTM Glass technology. This is a specially formulated coating that prevents the build up of soap and water spots. Install the surface with the ClearMaxTM label towards the inside of the shower. Please note that depending on the model, the glass may be coated on either one or both surfaces. For best results, squeegee the glass after each use and dry with a soft cloth. ENIGMA-X / SPARKLE Enclosure manual Ver 7 01/2017 2 B B A A C ! E IMPORTANT INFORMATION REGARDING THE INSTALLATION OF THIS SHOWER DOOR D PANEL DOOR F Right hand door installation shown as an example A Guide Rail Brackets must be firmly D Roller Guards must be postioned and attached to the wall. Installation into a secured within 1/16” of Upper Guide Rail. stud is strongly recommended. -

Ffontiau Cymraeg
This publication is available in other languages and formats on request. Mae'r cyhoeddiad hwn ar gael mewn ieithoedd a fformatau eraill ar gais. [email protected] www.caerphilly.gov.uk/equalities How to type Accented Characters This guidance document has been produced to provide practical help when typing letters or circulars, or when designing posters or flyers so that getting accents on various letters when typing is made easier. The guide should be used alongside the Council’s Guidance on Equalities in Designing and Printing. Please note this is for PCs only and will not work on Macs. Firstly, on your keyboard make sure the Num Lock is switched on, or the codes shown in this document won’t work (this button is found above the numeric keypad on the right of your keyboard). By pressing the ALT key (to the left of the space bar), holding it down and then entering a certain sequence of numbers on the numeric keypad, it's very easy to get almost any accented character you want. For example, to get the letter “ô”, press and hold the ALT key, type in the code 0 2 4 4, then release the ALT key. The number sequences shown from page 3 onwards work in most fonts in order to get an accent over “a, e, i, o, u”, the vowels in the English alphabet. In other languages, for example in French, the letter "c" can be accented and in Spanish, "n" can be accented too. Many other languages have accents on consonants as well as vowels. -

Percent R, X and Z Based on Transformer KVA
SHORT CIRCUIT FAULT CALCULATIONS Short circuit fault calculations as required to be performed on all electrical service entrances by National Electrical Code 110-9, 110-10. These calculations are made to assure that the service equipment will clear a fault in case of short circuit. To perform the fault calculations the following information must be obtained: 1. Available Power Company Short circuit KVA at transformer primary : Contact Power Company, may also be given in terms of R + jX. 2. Length of service drop from transformer to building, Type and size of conductor, ie., 250 MCM, aluminum. 3. Impedance of transformer, KVA size. A. %R = Percent Resistance B. %X = Percent Reactance C. %Z = Percent Impedance D. KVA = Kilovoltamp size of transformer. ( Obtain for each transformer if in Bank of 2 or 3) 4. If service entrance consists of several different sizes of conductors, each must be adjusted by (Ohms for 1 conductor) (Number of conductors) This must be done for R and X Three Phase Systems Wye Systems: 120/208V 3∅, 4 wire 277/480V 3∅ 4 wire Delta Systems: 120/240V 3∅, 4 wire 240V 3∅, 3 wire 480 V 3∅, 3 wire Single Phase Systems: Voltage 120/240V 1∅, 3 wire. Separate line to line and line to neutral calculations must be done for single phase systems. Voltage in equations (KV) is the secondary transformer voltage, line to line. Base KVA is 10,000 in all examples. Only those components actually in the system have to be included, each component must have an X and an R value. Neutral size is assumed to be the same size as the phase conductors. -

A Quotient Rule Integration by Parts Formula Jennifer Switkes ([email protected]), California State Polytechnic Univer- Sity, Pomona, CA 91768
A Quotient Rule Integration by Parts Formula Jennifer Switkes ([email protected]), California State Polytechnic Univer- sity, Pomona, CA 91768 In a recent calculus course, I introduced the technique of Integration by Parts as an integration rule corresponding to the Product Rule for differentiation. I showed my students the standard derivation of the Integration by Parts formula as presented in [1]: By the Product Rule, if f (x) and g(x) are differentiable functions, then d f (x)g(x) = f (x)g(x) + g(x) f (x). dx Integrating on both sides of this equation, f (x)g(x) + g(x) f (x) dx = f (x)g(x), which may be rearranged to obtain f (x)g(x) dx = f (x)g(x) − g(x) f (x) dx. Letting U = f (x) and V = g(x) and observing that dU = f (x) dx and dV = g(x) dx, we obtain the familiar Integration by Parts formula UdV= UV − VdU. (1) My student Victor asked if we could do a similar thing with the Quotient Rule. While the other students thought this was a crazy idea, I was intrigued. Below, I derive a Quotient Rule Integration by Parts formula, apply the resulting integration formula to an example, and discuss reasons why this formula does not appear in calculus texts. By the Quotient Rule, if f (x) and g(x) are differentiable functions, then ( ) ( ) ( ) − ( ) ( ) d f x = g x f x f x g x . dx g(x) [g(x)]2 Integrating both sides of this equation, we get f (x) g(x) f (x) − f (x)g(x) = dx. -

CONSONANT DIGRAPH- /Ch/ at the Beginning of Words
MINISTRY OF EDUCATION PRIMARY ENGAGEMENT PROGRAMME GRADE FOUR (4) WORKSHEET-TERM 3 SUBJECT: LITERACY WEEK 5: LESSON 1 Name: _______________________________ Date: __________________________ READING: CONSONANT DIGRAPH- /ch/ at the beginning of words FACTS/TIPS A consonant digraph is formed when two consonants work together to make one sound. Consonant digraph ch has three(3) different sounds. 1. /ch/ as in chair, chalk 2. /sh/ as in chef, 3. /k/ as in chaos Let us read these words. Listen to the beginning sound. 1. chalk 2. cheese 3. chip 4. champagne 2. chorus 5. Charmin 6. child 7. chew Read the text below Ms. Charmin and Mr. Philbert work at NAREI. Philbert is a driver and Charmin a clerk. One day our class teacher, Ms. Phillips took us on a field trip where we visited CARICOM, GUYSUCO AND NAREI. Our time spent at NAREI was awesome. We were shown the different departments. One brave pupil, Charlie, asked Ms. Charmin about the meaning of the word 78 NAREI. She told us that the word NAREI is an acronym that means National Agricultural Research and Extension Institute. Ms. Charmin then went on to explain to us the products that are made there. After our tour around the compound, she gave us huge packages of some of the products. We were so excited that we jumped for joy. We took a photograph with her, thanked her and then bid her farewell. On our way back to school we spoke of our day out. We all agreed that it was very informative. ON YOUR OWN Say the words below and listen to the beginning sound.