United States Patent Office Patented Dec
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ministerium Für Wirtschaft, Verkehr, Landwirtschft Und Weinbau Stiftstr. 9 55116 Mainz
Autobahnanschl. Mz.-Mombach Schiersteiner Brücke Rheinallee Zeichenerklärung W allaustraße Kaisertor Rhein DB Hauptbahnhof So finden Sie uns: Frauenlobstraße Rheinallee S S-Bahn Greif P Kaiserstraße fenklaustr Taxi Taxistand Forsterstraße Christus Adam-Karillon-Straße Ministerium für Wirtschaft, Verkehr, Kirche . H Bushaltestelle Hindenburgstraße Rheinufer Landwirtschft und Weinbau Ernst-Ludwig- Köln-Frankfurt-Kassel Kurfürstl. (Wiesbadener Kreuz) Parkplatz Stiftstr. 9 P von-Isenburg-StrSchloß H Kaiser- Hindenb. Str P Parkhaus/ Friedrich- . 55116 Mainz platz Str. P Landtag H Peter-Altmeier-Allee Tiefgarage Diether- Platz der Hauptverkehrswege Ministerium für Wirtschaft, Verkehr, . Landwirtschaft und Weinbau Leibnizstr Mainzer P Theodor-Heuss-Brücke Busverbindungen von Mainz Hbf Richtung Bauhofstraße: Republik Bauhofstraße . Linie: 6 Richtung Wiesbaden bis Haltestelle Bauhofstr. Stiftsstr Landesbank LRP, St. Peter Schieß - gartenstr H Linie: 65 Richtung Weisenau Haltestelle Bauhofstr. Landesbank LRP Bleiche -str. Eltzer Große Bleiche Fußweg von Mainz Hbf.: Boppstraße H Mitternacht Zeughausg Adam-Karillon-Straße Mitternachts Hof Brücken Kaiser-Wilhelm-Ring Flachs platz Ca. 10 Minuten über die Bahnhofstraße, Kaiserstr. Bauhofstr. Landes- Peters markt- Autobahn- Heidelbergerfaßgasse .museum str. str anschl. Hintere Reichklara Mz.-Mombach Rhabanusstr Kaiser- straße/ B 40 Löwen Bauerngasse P . MULEWF, Abt. 7 Frauenlobstraße Neubrunnenstraße eteng. g. Löhrstraße . H . Karmeliterstr hofstr Flachs Rheinstraße Wiesbaden markt = Ausschnitt siehe rechts Taxi Klarastraße . H Mainz Margar Christo Zanggasse fsg. Köln-Frankfurt H Innenstadt . Christofsstr Lotharstr Schusterstr (Wiesbadener Kreuz) H Hintere Bahn- Mombacher Straße g-Str Rathaus hofs- Schottstr . Römer- W.-Mainzer Str. S platz Schiersteiner Kreuz Emmeransstraße A 66 Taxi pass. P H str. Bahn Kolpin hofstr Große Bleiche Stadthaus- . DB Quintinsstraße Autobahnanschl. Wiesbaden-Erbenheim . Mittlere Bleiche Um Steingasse (W H bach Am Brand Am eisenauerMz.-W Brücke) Parcus straße Münster Kronberger . -

Planung Vernetzter Biotopsysteme Bereich Landkreis Mainz-Bingen
Planung Vernetzter Biotopsysteme Bereich Landkreis Mainz-Bingen Impressum Planung Vernetzter Biotopsysteme Bereiche Landkreis Mainz-Bingen und Kreisfreie Stadt Mainz Impressum Herausgeber Ministerium für Umwelt Rheinland-Pfalz, Kaiser-Friedrich-Str. 7, 55116 Mainz Landesamt für Umweltschutz und Gewerbeaufsicht Rheinland-Pfalz, Amtsgerichtsplatz 1, 55276 Oppenheim Bearbeitung Landesamt für Umweltschutz und Gewerbeaufsicht Rheinland-Pfalz, 55276 Oppenheim Dr. Rüdiger Burkhardt, Astrid Freese, Gerd Schwab Faunistisch-Ökologische Arbeitsgemeinschaft, Auf der Redoute 12, 54296 Trier Karsten Schnell, Achim Kiebel, Martin Schorr Beiträge Gesellschaft für Naturschutz und Ornithologie Rheinland-Pfalz e.V., Bachgasse 4, 56377 Nassau (Amphibien, Libellen, Vögel, Reptilien) IFÖNA GmbH, Mainzer Str. 94, 66121 Saarbrücken J. Mas, A. Saar, A. Busch (Tagfalter, Bestand, Teile von Kapitel B und C) GÖFA - Gesellschaft für ökologische Forschung, angewandten Natur- und Umweltschutz und Ökoprodukthandel mbH, W.-Th.-Römheld-Str. 34, 55130 Mainz Annette Lehna, Thomas Grunwald (Tagfalter) Graphische Realisation Faunistisch-Ökologische Arbeitsgemeinschaft, Trier Anja Knippel, Sandra Meier, Peter Haag Technische Realisation Faunistisch-Ökologische Arbeitsgemeinschaft, Trier Carmen Hertlein, Suse Bauschmid Fertigstellung Dezember 1999 Impressum Zitiervorschlag LfUG & FÖA (1998): Planung Vernetzter Biotopsysteme. Bereiche Landkreis Mainz-Bingen und Kreisfreie Stadt Mainz. Bearb.: Landesamt für Umweltschutz und Gewerbeaufsicht Rheinland-Pfalz & Faunistisch- -

Lunch at Bitburg Air Force Base, Bitburg, Germany, Josh/Rv (8) Box: 207
Ronald Reagan Presidential Library Digital Library Collections This is a PDF from our textual collections. Collection: Speechwriting, White House Office of: Research Office, 1981-1989 Folder: 05/05/1985 Remarks: Lunch at Bitburg Air Force Base, Bitburg, Germany, Josh/Rv (8) Box: 207 To see more digitized collections visit: https://reaganlibrary.gov/archives/digital-library To see all Ronald Reagan Presidential Library inventories visit: https://reaganlibrary.gov/document-collection Contact a reference archivist at: [email protected] Citation Guidelines: https://reaganlibrary.gov/citing ·:--... ""' --~. .- ~ \ \. ';> I ' -·' -\ .. -:,,..\ ' ~ 1: BITBlJFlG Bitburg, county capital of the Southern Eitel, located In the hills iroximity to the Benelux countries, Bitburg offers ideal settlement between the Kyll and the Nims rivers, has been for centuries the opportunities. Located at the intersection of several Federal high natural center of this predominantly agriculturally-oriented area. ways at only 27 KM from the central- and university city of Trier, the city will soon have good traffic connections with Antverp, Brussels, Among the many county Liege, and the Rhein-Main/Rhein-Neckar area via Federal Auto capitals of Rheinland Pfalz, the almost 2000-ynar bahn A-60. With the Beginning of the construction work on the new old Eifel city has an espe A-60 between German border and Bitburg, and the soon to follow cially interesting past. Age continuation up to Witllich (A-48) this traffic improvement plan has old East-West roads cross entered a decisive stage. here with the most impor tant North-South connec As the capital of Bitburg-Pr0m County, Bitburg is today the econo tion through! the Eifel from mic and cultural c'ent;r of the Southern Eitel. -

Die Neue Rheinbrücke Wiesbaden-Schierstein
Hessen Mobil Straßen- und Verkehrsmanagement Die neue Rheinbrücke Wiesbaden-Schierstein Wettbewerb und Entwurf Projekt1 19.10.2007 08:32 Seite 1 Fachthemen Eberhard Pelke DOI: 10.1002/stab.201310024 Alwin Dieter Die neue Rheinbrücke Wiesbaden-Schierstein – Wettbewerb und Entwurf Oft unterschätzt, stand die bestehende Rheinbrücke Wiesbaden- 1 Die vorhandene Rheinbrücke Wiesbaden-Schierstein Schierstein am Wendepunkt vom statischen zum fertigungsge- rechten Ingenieurbauwerk. Der Siegerentwurf des Realisierungs- Der erste Bedarfsplan für die Bundesfernstraßen der neuen wettbewerbes 2007 bestätigte die Grundlagen von Entwurf und Bundesrepublik Deutschland 1956 richtete ein besonderes Ausführung der Jahre 1959 bis 1962 und zieht Schlüsse aus der Augenmerk auf die Verknüpfung von Ballungs- und wich- Unter- und baulichen Erhaltung des bestehenden Bauwerks. Der tigen Wirtschaftsräumen beiderseits des Rheins. Nach dem Aufsatz beschreibt Entstehung des bestehenden Bauwerks, Kölner Ring und dem Bonner Ring war der Bau der Umge- wesentliche Instandsetzungsschritte, die sich daraus ableitende hungsstraße Mainz, d. h. dem Stadtring Mainz–Wiesbaden, Erfordernis zum Neubau und abschließend dessen Ausschrei- ein vordringliches Projekt. Zur Erschließung dieses Wirt- bungsentwurf. schaftsraums der Bundesländer Rheinland-Pfalz und Hessen waren zwei neue große Rhein- und eine Mainbrücke zu er- The new Rhine Bridge Wiesbaden-Schierstein, Germany – stellen. Competition and Design. The underestimated, existing Rhine Der Stadtring Mainz–Wiesbaden war durch die rechts- Bridge Wiesbaden-Schierstein was the watershed between rheinische Bundesstraße B 42 und die linksrheinische B 9 static and erection optimization. The winner of the design zu schließen und an den Rhein-Main-Schnellweg über die competition in 2007 for a new Rhine Bridge between Wies- B 26 mit der Autobahn Frankfurt–Basel (heute A 67) zu baden and Mainz confirms the basic design and construction verbinden. -

Empirica Forschung Und Beratung
empirica Forschung und Beratung Kurfürstendamm 234 10719 Berlin Tel. (030) 88 47 95-0 Fax (030) 88 47 95-17 www.empirica-institut.de [email protected] Fortschreibung der Sozialraumanalyse Mainz Im Auftrag der Landeshauptstadt Mainz, Dezernat für Soziales, Kinder, Jugend, Schule und Gesundheit Verfasser: Ulrich Pfeiffer, Andreas Vater, Kristina Kröger, Projektnummer: 2009064 Julia Kemper, Sebastian Scholze Berlin, 13. Juli 2012 Inhaltsverzeichnis INHALTSVERZEICHNIS I KARTENVERZEICHNIS V TABELLENVERZEICHNIS IX ABBILDUNGSVERZEICHNIS XI I. HINTERGRUND UND METHODISCHES VORGEHEN 1 1. Aufgabenstellung 1 2. Aufbau der Sozialraumanalyse 2 3. Gebietsabgrenzung und räumliche Bezugsebene 2 II. DESKRIPTIVE ANALYSE 6 1. Vorbemerkung 6 2. Indikatorenbereich Flächennutzung 7 2.1 Nutzungsmischung: Wohnen, Gewerbe, Verkehr, Grün- und Freizeitfläche 7 2.2 Bevölkerungsdichte 13 3. Indikatorenbereich Demographie 16 3.1 Bevölkerung nach Altersstrukturen 17 3.2 Anteil und Altersstruktur der Bevölkerung mit Migrationshintergrund 25 3.2.1 Anteil der Einwohner mit Migrationshintergrund an der Gesamtbevölkerung 25 3.2.2 Anteile der Einwohner mit Migrationshintergrund an den jeweiligen Altersgruppen 31 3.2.3 Altersstruktur der Einwohner mit Migrationshintergrund 43 3.2.4 Spannungspotential 55 3.3 Bevölkerungsentwicklung 60 3.3.1 Natürliche Bevölkerungsentwicklung 60 3.3.2 Wanderungsbedingte Bevölkerungsentwicklung 67 3.3.2.1 Wanderungsbewegung über die Stadtgrenzen (Außenwanderung) 67 3.3.3 Wanderungsbewegung innerhalb der Stadtgrenzen (Binnenwanderung) 74 Sozialraumanalyse Mainz - i - empirica 3.3.3.1 Mobilitätsziffer 81 3.3.4 Bevölkerungsentwicklung insgesamt 85 3.3.5 Vertiefungsbereich Kinder und Jugendliche 93 3.3.6 Vertiefungsbereich Ältere Menschen 102 3.3.6.1 Altenquotient und Alt-Jung-Quotient 102 3.3.6.2 Altersgruppen Senioren 108 4. Indikatorenbereich Haushaltsstruktur und Familie 114 4.1 Haushalte nach Haushaltsgröße und Haushaltstyp 114 4.2 Einpersonenhaushalte 117 4.3 Familienhaushalte 123 4.3.1 Paarhaushalte mit Kindern 127 4.3.2 Alleinerziehende 132 5. -

14 Gaerten Der Erinnerung.Pdf
Inhalt Vorwort 5 Friedhöfe im Stadtgebiet Mainz 6-7 Hauptfriedhof Mainz 8-9 Alter jüdischer Friedhof »Am Judensand« und Neuer jüdischer Friedhof Mainz 10 Friedhof Mainz-Bretzenheim und jüdische Friedhöfe Mainz-Bretzenheim 11 Friedhof Mainz-Gonsenheim 12-13 Waldfriedhof Mainz-Mombach 14-15 Bezirksfriedhof Mainz-West 16 Friedhof Mainz-Drais 17 Friedhof Mainz-Laubenheim 18-19 Friedhof Mainz-Finthen 20 Friedhof Mainz-Marienborn 21 Alter Friedhof Mainz-Weisenau und jüdischer Friedhof Mainz-Weisenau 22 Neuer Friedhof Mainz-Weisenau 23 Friedhof Mainz-Hechtsheim und jüdischer Friedhof Mainz-Hechtsheim 24-25 Friedhof Mainz-Ebersheim und jüdischer Friedhof Mainz-Ebersheim 26-27 Vorwort Liebe Mitbürgerinnen und Mitbürger, 5 liebe Besucherinnen und Besucher der Mainzer Friedhöfe, Friedhöfe haben eine eigene Geschichte. Mit dieser Broschüre laden wir Sie ein, der Sie sprechen eine eigene Sprache. Vielfalt und zugleich einer Einheit im Zusam- Als aufgeschlagenes Buch der Stadtgeschich- menwirken der Friedhöfe im Mainzer Stadt- te präsentieren sich auch die Friedhöfe im gebiet neu zu begegnen. Lassen Sie sich Mainzer Stadtgebiet. von der Einzigartigkeit und der besonderen Atmosphäre eines jeden Bestattungsortes Vierzehn kommunale Friedhöfe und sieben einnehmen, und überzeugen Sie sich von den jüdische Friedhöfe lassen uns das Thema Tod Möglichkeiten, inmitten eines gemeinsamen und Vergänglichkeit in den Mittelpunkt rücken Ortes der Trauer der individuellen Persönlich- und laden uns zur eingehenden Beschäfti- keit Form zu verleihen. Wolfgang Reichel gung mit der Denkmalkultur und der Land- schaftsarchitektur ein. Friedhöfe als Orte der Trauer, als Orte des Gedenkens, aber auch als Orte der Begegnung und des Lebens bieten uns ein natürliches Umfeld zur Besinnung. Prachtvolle alte Baumbestände beeindrucken den Besucher und verleihen unseren Friedhöfen die Funktion einer Grünanlage mit hohem Erholungswert. -

Sewage Sludge Management in Germany Authors: Dipl.-Ing
Sewage sludge management in Germany Authors: Dipl.-Ing. Benjamin Wiechmann Dipl.-Ing. Claudia Dienemann Dr. Christian Kabbe M. Sc. Simone Brandt Dr. Ines Vogel Dr. Andrea Roskosch Order brochures address: Umweltbundesamt c/o GVP Postfach 30 03 61 | 53183 Bonn | Germany The brochure is free. Telephone service: +49 (0)3 40 21 03-66 88 Fax service: +49 (0)3 40 21 04-66 88 www.umweltbundesamt.de Sewage sludge management in Germany Publisher: Umweltbundesamt (UBA) | Postfach 1406 | 06844 Dessau-Roßlau | Germany Contents Contents Preface 3 7 Sewage sludge quantities, management 42 and recycling 1 Introduction 4 What is sewage sludge? 4 8 Footing the bill for sewage sludge 50 Where does sewage sludge occur? 5 management 2 Composition of sewage sludge 7 9 The way forward 52 Heavy metals in sewage sludge 9 Organic compounds in sewage sludge 11 10 Illustrations 58 Pathogens and health hazards arising 12 from e.g. EHEC 11 Tabels 58 Pharmaceutical drug residues in sewage 14 sludge 12 Abbreviations 60 3 Sludge treatment 16 13 Acknowledgements 62 4 Thermal sludge treatment 23 14 Bibliography 62 Mono-incineration 23 Co-incineration 25 15 Appendix I 68 5 Sewage sludge use in the agricultural 29 16 Appendix II 82 sector Sewage sludge management legislation 82 Nutrients in sewage sludge 30 Sewage sludge pollutants 31 17 Appendix III 87 Pros and cons of using sewage sludge as 34 Heavy metals in sewage sludge 87 a fertilizer 18 Appendix IV 88 6 Phosphorous recovery 34 Phosphorous recovery potential and 36 processes Cost efficient phosphorous recycling in 38 Germany 2 Preface Preface Germany’s municipal sewage treatment plants However, sewage sludge is set to take on grea- generate some two million tons of dry sewage ter importance as a raw material, mainly due sludge annually, with the proportion of ther- to the increased concentrations of phospho- mally treated sewage sludge increasing from rous it contains. -
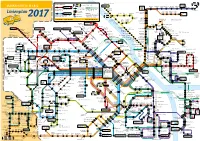
Liniennetzplan2017.Pdf
Wi.-Schierstein Tierpark Zeichenerklärung Fasanerie 33 Zeilstraße 9 alternative Endhaltestelle Fasanerie- Oderstraße 9 straße Platz der Michels- Riederberg- Dürer- Westerwald- Albrecht- Holbein- 33 Alt 68 Berliner Platz 68 S8 berg straße platz straße Dürer-Schule straße Klarenthal Eishaus Haltestelle nur Dt. Einheit in eine Richtung Neckarstraße Buslinie mit Haltestelle, Liniennummer S-Bahnlinie mit Haltestelle 28 N7 und Endhaltestelle Stollenweg Umsteige- und Liniennummer Rathaus haltestelle Adler- Roth- Ruhberg- Wolken- Schwalbacher Straße/ straße straße straße bruch Linienplan Reichsapfel- Saarbrücker Alte Hagenauer Nordfriedhof 52 Bahnstraße 52 LuisenForum Allee Schmelze Straße Krankenhaus auszugsweise RegionalBahn / straße 6 Straßenbahn mit Haltestelle, Linien- Regional-Express mit Halte- N7 nummer und Endhaltestelle P+R Park and Ride stelle und Liniennummer Zeilstraße 9 2017 Äppelallee- Landes- Info-Hotline: 06131-12 77 77 www.mvg-mainz.de Center bibliothek Abraham-Lincoln- Floßhafen/ Carl- Friedrich- Straße/Uniserve Wiesbaden Dow Corning Bosch- Bergius- Adelheidstraße Straße Straße Gültig ab 11. Dezember 2016 Mombacher Schiersteiner Landeshaus Richtung 76 78 Kreisel Brücke Berliner Budenheim John-F.-Kennedy-Straße 9 Straße Frieden- Richtung straße In der Sauerwiese Im Hahn Ingelheim Chem. Fabrik Abzw. Dalheimer real-Markt Wi.- Statistisches Im Herzen Bahnhof 68 Wildpark 6 62 92 Am Polygon 60 Waldfriedhof 61 Kreussler Hauptbahnhof Hauptstraße Waldfriedhof Wiese Mombach Biebrich Bundesamt N7 Am Hochfeld Rheinhütte A.- Kirche Mz.-Mombach -

Restructuring the US Military Bases in Germany Scope, Impacts, and Opportunities
B.I.C.C BONN INTERNATIONAL CENTER FOR CONVERSION . INTERNATIONALES KONVERSIONSZENTRUM BONN report4 Restructuring the US Military Bases in Germany Scope, Impacts, and Opportunities june 95 Introduction 4 In 1996 the United States will complete its dramatic post-Cold US Forces in Germany 8 War military restructuring in ● Military Infrastructure in Germany: From Occupation to Cooperation 10 Germany. The results are stag- ● Sharing the Burden of Defense: gering. In a six-year period the A Survey of the US Bases in United States will have closed or Germany During the Cold War 12 reduced almost 90 percent of its ● After the Cold War: bases, withdrawn more than contents Restructuring the US Presence 150,000 US military personnel, in Germany 17 and returned enough combined ● Map: US Base-Closures land to create a new federal state. 1990-1996 19 ● Endstate: The Emerging US The withdrawal will have a serious Base Structure in Germany 23 affect on many of the communi- ties that hosted US bases. The US Impact on the German Economy 26 military’syearly demand for goods and services in Germany has fal- ● The Economic Impact 28 len by more than US $3 billion, ● Impact on the Real Estate and more than 70,000 Germans Market 36 have lost their jobs through direct and indirect effects. Closing, Returning, and Converting US Bases 42 Local officials’ ability to replace those jobs by converting closed ● The Decision Process 44 bases will depend on several key ● Post-Closure US-German factors. The condition, location, Negotiations 45 and type of facility will frequently ● The German Base Disposal dictate the possible conversion Process 47 options. -

Anfahrt Mainz-Rheinallee
Rheinallee 97-101 55118 Mainz Telefon 06131 967-0 Telefax 06131 967-310 [email protected] Anfahrtsbeschreibung Das Hauptgebäude des Landesamtes für Soziales, Jugend und Versorgung befindet sich in unmittelbarer Nähe des Zollhafens. Anfahrt mit öffentlichen Verkehrsmitteln Das Hauptgebäude in der Rheinallee ist ab Mainz Hauptbahnhof mit den nachfolgend genannten Bus- und Straßenbahnlinien zu erreichen: Buslinien: Linie 76 Richtung Mombacher Kreisel: 5 Stationen bis Mainstraße/Bewegungszentrum Mainz Linie 67 Richtung Wallaustraße: 6 Stationen bis Wallaustraße (Endstation) Linie 62 Richtung Mainz-Gonsenheim: 9 Stationen bis Sömmerringstraße Linie 63 Richtung Mainz-Mombach: 9 Stationen bis Sömmerringstraße Die Fahrt der Linien 62 und 63 verläuft zunächst durch die Innenstadt. Von den genannten Haltestellen ist es jeweils nur ein kurzer Fußweg zum Dienstgebäude. Straßenbahnlinien: Linie 50 Richtung Mainz-Finthen: 2 Stationen bis zur Goethestraße Linie 51 Richtung Mainz-Finthen: 2 Stationen bis zur Goethestraße Von der Goethestraße bis zum Dienstgebäude sind es circa 10 Minuten Fußweg. Anfahrt mit dem Auto 1. Aus Richtung Frankfurt/Main: A 66 Frankfurt-Wiesbaden, Richtung Rüdesheim. Am Schiersteiner Kreuz auf die A 643, Richtung Mainz. Ausfahrt Mainz-Mombach/Budenheim – siehe Pkt. 6. 2. Aus Richtung Frankfurt/Flughafen; Darmstadt: A 60, Richtung Mainz. Am Autobahndreieck Mainz auf die A 643, Richtung Wiesbaden/Frankfurt. Ausfahrt Mainz-Mombach/Budenheim – siehe Pkt. 6. 3. Aus Richtung Koblenz: A 60 Bingen-Mainz, Richtung Mainz. Am Autobahndreieck Mainz auf die A 643, Richtung Wiesbaden/Frankfurt. Ausfahrt Mainz-Mombach/Budenheim – siehe Pkt. 6. 4. Aus Richtung Ludwigshafen: A 63 Kaiserslautern-Mainz, Richtung Mainz. Am Autobahnkreuz Mainz auf die A 60, Richtung Bingen-Koblenz. Am Autobahndreieck Mainz auf die A 643, Richtung Wiesbaden/Frankfurt. -

Rechtsverordnung Mainzer Sand Teil II
315-183 Rechtsverordnung über das Naturschutzgebiet "Mainzer Sand Teil II" Stadt Mainz und Landkreis Mainz-Bingen vom 21. März 1997 (Staatsanzeiger für Rheinland-Pfalz vom 14. April 1997, Nr. 11, S. 434) Auf Grund des § 21 des Landespflegegesetzes (LPflG) in der Fassung vom 5. Februar 1979 (GVBl. S. 36), zuletzt geändert durch das Zweite Landesgesetz zur Änderung des Landespflegegesetzes vom 14. Juni 1994 (GVBl. S. 280), wird verordnet: § 1 Bestimmung zum Naturschutzgebiet Das in § 2 näher beschriebene und in der beigefügten Karte gekennzeichnete Gebiet wird zum Natur- schutzgebiet bestimmt; es trägt die Bezeichnung "Mainzer Sand Teil II". § 2 Größe und Grenzverlauf (1) Das Naturschutzgebiet ist etwa 100 ha groß; es umfasst Teile der Gemarkungen Mombach und Gonsenheim, kreisfreie Stadt Mainz, und Teile der verbandsfreien Gemeinde Buden- heim, Landkreis Mainz-Bingen. (2) Die Grenze des Gebietes verläuft, am westlichen Ortsrand von Mainz-Mombach an der L 423 beginnend, wie folgt: Vom nordöstlichen Eckpunkt des Flurstücks 46/3 in der Gemarkung Mombach, Flur 6, der L 423 nach Westen folgend bis zur Straße "Schwarzer Weg". Von dort folgt sie der Westsei- te des Wirtschaftsweges, der westlich entlang der vorgenannten Straße in die Gemarkung Budenheim führt. Sie begleitet diesen bis zum Flurstück 459 in der Flur 9 dieser Gemarkung, folgt der östlichen Grenze dieses Flurstücks bis zur Straße "In den Vierzehn Morgen", be- gleitet diese nach Süden bis zum Flurstück 255/5, folgt dessen Nord-, Ost- und Südgrenze, dann den Ostgrenzen des Flurstücks 440, 400/2 und 400/1 bis zum Weg Flurstück 175, be- gleitet dessen Südseite nach Osten in die Flur 10 bis zum Weg 173/2. -

Radrouten Rheinhessen.Pdf
EINLEITUNG RADROUTEN RHEINHESSEN E-BIKE Gräselberg 200 A 66 Okriftel Wisper B 260 A 66 Erbenheim Silbersee Romantischer Rhein 300 200 Rheingau-Taunus Schierstein Delkenheim RADROUTEN 500 B 42 580 Kloster A 3 Willkommen im Land der 1000 Hügel 400 Parkfeld 3 00 Kiedrich 00 4 Eberbach Wiesbaden Massenheim Weilbach 4 Walluf Schloss 4 00 Stephanshausen 0 Niederheimbach0 Eltville Mönchwaldsee Welcome to the land of a thousand hills Biebrich 100 0 0 Biebrich B 455 RHEINHESSEN B 9 R 5 B 40 Eddersheim h e Burg am Rhein A 643 i 3 538 300 Hallgarten n 00 Wicker 00 B 42 Eltville A 3 Rund 550 entspannte bis sportliche Kilometer Radwegenetz durchziehen 2 500 Mainz-Amöneburg B 519 4 0 0 in RADELN ZWISCHEN B 40 Flörsheim am a das Hügelland am Rhein. Im größten Weinanbaugebiet Deutschlands gibt ein M Hattenheim Rh 00 00 B 43 1 es neben guten Weinen so einige Entdeckungen zu machen. Hier kommen Trechtingshausen 3 Budenheim Mombach A 671 Main RHEIN UND REBEN Mainz-Kastel Hochheim am Main Raunheimer Genussradler wie Ambitionierte garantiert auf ihre Kosten. Marienthal Oestrich-Winkel Waldsee Abtei St. Johannisberg Hartenberg Sanfte Hügel und Täler, weite Rebenmeere, großartige Panoramen und 617 Reduit A 67 00 Schloss Winkel Raunheim 3 Hildegard A 60 Gonsenheim Neustadt der Rhein mit den namhaften Weinhängen der Rheinterrasse erwarten Aulhausen Johannisberg Sandlac he lte Heidesheim Gustavsburg Main Lindensee 0 Windeck 0 A 2 Münchfeld Falkenberg Sie. Im lebendigen Städteviereck von Mainz, Worms, Alzey und Bingen Assmannshausen Geisenheim Ingelheim B 40 (Keramag) 432 Rüdesheim 1 Eibingen Frei- L 419 Mainz Dom St.