MITK-Modelfit: a Generic Open-Source Framework for Model Fits and Their Exploration in Medical Imaging – Design, Implementatio
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Qualitative Comparison of Conventional and Oblique MRI for Detection of Herniated Spinal Discs
Qualitative Comparison of Conventional and Oblique MRI for Detection of Herniated Spinal Discs Doug Dean Final Project Presentation ENGN 2500: Medical Image Analysis May 16, 2011 Tuesday, May 17, 2011 Outline • Review of the problem presented in the paper: “A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen” (Authors: Shim JH, Park CK, Lee JH, et. al) • Approach to solve this problem • Data Acquisition • Analysis Methods • Results • Discussion/Conclusions Tuesday, May 17, 2011 Review of Problem • Difficult to identify herniated discs and spinal stenosis using conventional (2D) MRI techniques • These conventional methods result in patients condition being misdiagnosed. “Conventional MRI”: Images acquired along one of three anatomical planes Tuesday, May 17, 2011 3D reconstructive CT Axial, T2-weighted Image: Image shows that the Cervical Foramen is cervical foramina are directed at 45 degrees directed downward around with respect to coronal 10-15 degrees with plane. respect to axial plane Tuesday, May 17, 2011 Orientation of Images Conventional MRI: Sagittal Protocol Oblique MRI: Sagittal Protocol Tuesday, May 17, 2011 Timeline • Week 1 (4/11-4/16) • Work on developing MR imaging protocols and sequences • Recruit volunteers (~4-5 volunteers) • Week 2 (4/17-4/23) • Continue developing imaging sequences and begin data acquisition at the MRI facility • Assisted by Dr. Deoni • Week 3&4 (4/24-5/7) • Continue developing and testing sequence • 4/27/2011: Acquisition of first subject • Mid Project Presentation: Describe the imaging protocols, present data that had been acquired from previous week, describe what still needs to be done. -

Survey of Databases Used in Image Processing and Their Applications
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 1 ISSN 2229-5518 Survey of Databases Used in Image Processing and Their Applications Shubhpreet Kaur, Gagandeep Jindal Abstract- This paper gives review of Medical image database (MIDB) systems which have been developed in the past few years for research for medical fraternity and students. In this paper, I have surveyed all available medical image databases relevant for research and their use. Keywords: Image database, Medical Image Database System. —————————— —————————— 1. INTRODUCTION Measurement and recording techniques, such as electroencephalography, magnetoencephalography Medical imaging is the technique and process used to (MEG), Electrocardiography (EKG) and others, can create images of the human for clinical purposes be seen as forms of medical imaging. Image Analysis (medical procedures seeking to reveal, diagnose or is done to ensure database consistency and reliable examine disease) or medical science. As a discipline, image processing. it is part of biological imaging and incorporates radiology, nuclear medicine, investigative Open source software for medical image analysis radiological sciences, endoscopy, (medical) Several open source software packages are available thermography, medical photography and for performing analysis of medical images: microscopy. ImageJ 3D Slicer ITK Shubhpreet Kaur is currently pursuing masters degree OsiriX program in Computer Science and engineering in GemIdent Chandigarh Engineering College, Mohali, India. E-mail: MicroDicom [email protected] FreeSurfer Gagandeep Jindal is currently assistant processor in 1.1 Images used in Medical Research department Computer Science and Engineering in Here is the description of various modalities that are Chandigarh Engineering College, Mohali, India. E-mail: used for the purpose of research by medical and [email protected] engineering students as well as doctors. -
PDF File, 212 KB
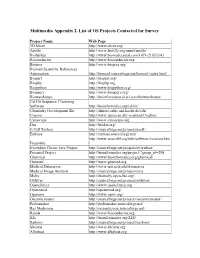
Multimedia Appendix 2. List of OS Projects Contacted for Survey Project Name Web Page 3D Slicer http://www.slicer.org/ Apollo http://www.fruitfly.org/annot/apollo/ Biobuilder http://www.biomedcentral.com/1471-2105/5/43 Bioconductor http://www.bioconductor.org Biojava http://www.biojava.org/ Biomail Scientific References Automation http://biomail.sourceforge.net/biomail/index.html Bioperl http://bioperl.org/ Biophp http://biophp.org Biopython http://www.biopython.org/ Bioquery http://www.bioquery.org/ Biowarehouse http://bioinformatics.ai.sri.com/biowarehouse/ Cd-Hit Sequence Clustering Software http://bioinformatics.org/cd-hit/ Chemistry Development Kit http://almost.cubic.uni-koeln.de/cdk/ Coasim http://www.daimi.au.dk/~mailund/CoaSim/ Cytoscape http://www.cytoscape.org Das http://biodas.org/ E-Cell System http://sourceforge.net/projects/ecell/ Emboss http://emboss.sourceforge.net/ http://www.ensembl.org/info/software/versions.htm Ensemble l Eviewbox Dicom Java Project http://sourceforge.net/projects/eviewbox/ Freemed Project http://bioinformatics.org/project/?group_id=298 Ghemical http://www.bioinformatics.org/ghemical/ Gnumed http://www.gnumed.org Medical Dataserver http://www.mii.ucla.edu/dataserver Medical Image Analysis http://sourceforge.net/projects/mia Moby http://biomoby.open-bio.org/ Olduvai http://sourceforge.net/projects/olduvai/ Openclinica http://www.openclinica.org Openemed http://openemed.org/ Openemr http://www.oemr.org/ Oscarmcmaster http://sourceforge.net/projects/oscarmcmaster/ Probemaker http://probemaker.sourceforge.net/ -

Whole Body Computed Tomography with Advanced Imaging Techniques: a Research Tool for Measuring Body Composition in Dogs
Hindawi Publishing Corporation Journal of Veterinary Medicine Volume 2013, Article ID 610654, 6 pages http://dx.doi.org/10.1155/2013/610654 Research Article Whole Body Computed Tomography with Advanced Imaging Techniques: A Research Tool for Measuring Body Composition in Dogs Dharma Purushothaman,1 Barbara A. Vanselow,2 Shu-Biao Wu,1 Sarah Butler,3 and Wendy Yvonne Brown1 1 School of Environmental and Rural Science, Department of Animal Science, University of New England, Armidale, NSW 2351, Australia 2 NSW Department of Primary Industries, Beef Industry Centre, University of New England, Armidale, NSW 2351, Australia 3 North Hill Vet Clinic, Armidale, NSW 2350, Australia Correspondence should be addressed to Wendy Yvonne Brown; [email protected] Received 6 May 2013; Revised 14 September 2013; Accepted 17 September 2013 Academic Editor: Juan G. Chediack Copyright © 2013 Dharma Purushothaman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The use of computed tomography (CT) to evaluate obesity in canines is limited. Traditional CT image analysis is cumbersome and uses prediction equations that require manual calculations. In order to overcome this, our study investigated the use of advanced image analysis software programs to determine body composition in dogs with an application to canine obesity research. Beagles and greyhounds were chosen for their differences in morphology and propensity to obesity. Whole body CT scans with regular intervals were performed on six beagles and six greyhounds that were subjected to a 28-day weight-gain protocol. -

Evaluation of DICOM Viewer Software for Workflow Integration in Clinical Trials
Evaluation of DICOM Viewer Software for Workflow Integration in Clinical Trials Daniel Haak1*, Charles-E. Page, Klaus Kabino, Thomas M. Deserno Department of Medical Informatics, Uniklinik RWTH Aachen, 52057 Aachen, Germany ABSTRACT The digital imaging and communications in medicine (DICOM) protocol is nowadays the leading standard for capture, exchange and storage of image data in medical applications. A broad range of commercial, free, and open source software tools supporting a variety of DICOM functionality exists. However, different from patient’s care in hospital, DICOM has not yet arrived in electronic data capture systems (EDCS) for clinical trials. Due to missing integration, even just the visualization of patient’s image data in electronic case report forms (eCRFs) is impossible. Four increasing levels for integration of DICOM components into EDCS are conceivable, raising functionality but also demands on interfaces with each level. Hence, in this paper, a comprehensive evaluation of 27 DICOM viewer software projects is performed, investigating viewing functionality as well as interfaces for integration. Concerning general, integration, and viewing requirements the survey involves the criteria (i) license, (ii) support, (iii) platform, (iv) interfaces, (v) two- dimensional (2D) and (vi) three-dimensional (3D) image viewing functionality. Optimal viewers are suggested for applications in clinical trials for 3D imaging, hospital communication, and workflow. Focusing on open source solutions, the viewers ImageJ and MicroView are superior for 3D visualization, whereas GingkoCADx is advantageous for hospital integration. Concerning workflow optimization in multi-centered clinical trials, we suggest the open source viewer Weasis. Covering most use cases, an EDCS and PACS interconnection with Weasis is suggested. -

Transforming the Medical Imaging Workflow
Transforming the Medical Imaging Workflow: How Mac systems and open source software combine to make full-featured diagnostic imaging solutions affordable for today’s radiologist. By Roger Katen, M.D. San Francisco, CA White Paper 2 Transforming the Medical Imaging Workflow Contents Page 3 Executive Summary Page 5 The New Realities of Medical Imaging The Digital Data Explosion The Rise of Imaging Workstations A Cost Conundrum Page 8 A Compelling Alternative: The Mac and OsiriX Workstation OsiriX: Full-Featured, Open Source Mac OS X and Mac Systems A New Solution for a New Reality Page 10 Mac Systems and OsiriX in the Radiology Workflow Image Generation Equipment Picture Archiving and Communications Systems (PACS) Creating a PACS Archive with OsiriX Database Sharing and Autorouting Storage System Page 16 Building an Imaging Workstation Environment with OsiriX Working with Large Datasets Supporting Medical-Quality Displays Requirements for High-Performance Processors Accelerating Image Retrieval Page 19 Enabling the Imaging Workflow Options for Advanced Visualization Starting and Sending a New DICOM Series to PACS Emailing and Printing Images Integrating with Hospital and Radiology Information Systems Exploring Alternatives to Windows RIS Systems Page 24 Collaborating Beyond the Office with OsiriX Virtual Private Networks Remote Visualization via Apple Remote Desktop Citrix/Windows Terminal Services Teleradiology Collaboration iChat and iChat Theater Page 28 Conclusion White Paper 3 Transforming the Medical Imaging Workflow Executive Summary Today more than ever, the healthcare industry is reaping the benefits of advances in diagnostic medical imaging. Dramatic breakthroughs in higher-resolution CT, MRI, ultrasound, and interventional technologies have enabled healthcare providers to deliver more informed diagnoses, pursue more effective treatments, collaborate more easily with colleagues, and communicate more clearly with patients. -

Automated 3D Visualization of Brain Cancer
AUTOMATED 3D VISUALIZATION OF BRAIN CANCER AUTOMATED 3D VISUALIZATION OF BRAIN CANCER By MONA AL-REI, MSc. A Thesis Submitted to the School of Graduate Studies In Partial Fulfillment of the Requirements for the Degree Master of eHealth Program McMaster University @ Copyright by Mona Al-Rei, June 2017 McMaster University Master of eHealth (2017) Hamilton, Ontario TITLE: 3D Brain Cancer Visualization. AUTHOR: Mona Al-Rei. SUPERVISOR: Dr. Thomas Doyle. SUPERVISRORY COMMITTEE: Dr. Reza Samavi, Dr. David Koff. NUMBER OF PAGES: xvii, 119. ii To my beloved and wounded country Yemen iii Abstract Three-dimensional (3D) visualization in cancer control has seen recent progress due to the benefits it offers to the treatment, education, and understanding of the disease. This work identifies the need for an application that directly processes two-dimensional (2D) DICOM images for the segmentation of a brain tumor and the generation of an interactive 3D model suitable for enabling multisensory learning and visualization. A new software application (M-3Ds) was developed to meet these objectives with three modes of segmentation (manual, automatic, and hybrid) for evaluation. M-3Ds software was designed to mitigate the cognitive load and empower health care professionals in their decision making for improved patient outcomes and safety. Comparison of mode accuracy was evaluated. Industrial standard software programs were employed to verify and validate the results of M-3Ds using quantitative volumetric comparison. The study determined that M-3Ds‘ hybrid mode was the highest accuracy with least user intervention for brain tumor segmentation and suitable for the clinical workflow. This paper presents a novel approach to improve medical education, diagnosis, treatment for either surgical planning or radiotherapy of brain cancer. -

MRI and CT Fusion in Stereotactic Electroencephalography: a Literature Review
applied sciences Systematic Review MRI and CT Fusion in Stereotactic Electroencephalography: A Literature Review Jaime Perez 1,* , Claudia Mazo 1,2,3 , Maria Trujillo 1 and Alejandro Herrera 1,4 1 Multimedia and Computer Vision Group, Universidad del Valle, Cali 760001, Colombia; [email protected] (C.M.); [email protected] (M.T.); [email protected] (A.H.) 2 UCD School of Computer Science, University College Dublin, Dublin 4, Ireland 3 CeADAR Ireland’s Centre for Applied AI, Dublin 4, Ireland 4 Clinica Imbanaco Grupo Quironsalud, Cali 760001, Colombia * Correspondence: [email protected] Abstract: Epilepsy is a common neurological disease characterized by spontaneous recurrent seizures. Resection of the epileptogenic tissue may be needed in approximately 25% of all cases due to ineffec- tive treatment with anti-epileptic drugs. The surgical intervention depends on the correct detection of epileptogenic zones. The detection relies on invasive diagnostic techniques such as Stereotactic Electroencephalography (SEEG), which uses multi-modal fusion to aid localizing electrodes, using pre-surgical magnetic resonance and intra-surgical computer tomography as the input images. More- over, it is essential to know how to measure the performance of fusion methods in the presence of external objects, such as electrodes. In this paper, a literature review is presented, applying the methodology proposed by Kitchenham to determine the main techniques of multi-modal brain image fusion, the most relevant performance metrics, and the main fusion tools. The search was conducted using the databases and search engines of Scopus, IEEE, PubMed, Springer, and Google Citation: Perez, J.; Mazo, C.; Trujillo, Scholar, resulting in 15 primary source articles. -

Segmentation Tools Frederic Cervenansky What Is Open-Source
Through the forest of open source segmentation tools Frederic Cervenansky What is Open-Source Open source doesn’t just mean access to source code 1 - Free Redistribution (no restriction from selling or giving away the software as a component) 2 - Source Code ( as well as compiled form) 3 - Derived Works (copyleft license) 4 - Integrity of The Author's Source Code (users have a right to know who is the original author) 5 – Distribution of License (no need of a third license) 6 and more What criteria to discriminate software? Usability Code Langage Segmentation methods Plugins – Extensions Organ/structure specificity Software Organs specific Code Segmentation Plugins Citations* Language Methods ImageJ no Java Automatic Yes 723 Fiji No ( Cell) Java Automatic Yes 1254 Freesurfer Brain C++, bash Automatic no 470 ITK no C++ Automatic Yes-no 512 Slicer3D No (Brain) C++ Manual- Automatic Yes 75 FSL Brain C++, bash Automatic No 690 SPM Brain matlab Automatic Yes 7574 pubmed … --- --- --- --- --- *: JAVA Bio-imaging Plugins system User friendly IMAGEJ 2D and 3D limitation No hierarchy in plugins ImgLib (n-dimensional, repackaging, sharing improvement) KMeans Color Quantization Color Picker Fiji Marching IMAGEJ2 Icy Threshold Learning Active Contours Squassh KMeans Clustering Texture Level Set Maximum Entropy Threshold HK-Means Graph Cut Maximum Multi Entropy Threshold Active Cells Bad Indexation: Seeded Region Growing Toolbox dithering as Snake. segmenation tool Brain Organ specific Fully automatic FreeSurfer Allow to restart at divergent steps. Both available Fully automatic through VIP Catalog of tools FSL BET ( Brain Extraction) FAST (GM and WM automated Segmentation). SPM Modified gaussian mixture model Need a matlab license. -

Neuroimaging DICOM and Nifti Primer
Neuroimaging DICOM and NIfTI Primer Overview Topic Description File Extensions DICOM: .dcm; NIfTI: .nii (uncompressed), nii.gz (compressed) MIME Type application/dicom Structure - DICOM: A header with demographic information about the patient and metadata about the image(s), followed by image data sets. - NIfTI: Contains image data set with an option JSON file that contains metadata (if converted using dcm2niix). Versions NIFTI-1, NIFTI-2 (not bitwise compatible) Primary fields or areas of use Neuroimaging Source and affiliation - NIfTI: The National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke are joint sponsors of the initiative to define and maintain this format. - DICOM: The copyright for the DICOM Standard is held by the National Electrical Manufacturers Association (NEMA) on behalf of the DICOM Standards Committee, administered by the Medical Imaging Technology Association (MITA) Metadata standards - DICOM: Header consists of standardized series of tags - NIfTI: Header consists of standardized series of tags, but few relate to file administration (eg., Suggested Citation: Moore, Michael; Patterson, Brandon; Samuel, Sara; Sheridan, Helenmary; Sorensen, Chris. (2020). Neuroimaging DICOM and NIfTI Data Curation Primer. http://hdl.handle.net/11299/216582 This work was created as part of the “Specialized Data Curation” Workshop #3 held at Washington University in St.Louis, MO on November 5-6, 2019. These workshops have been generously funded by the Institute of Museum and Library Services -

Our Lady of Lourdes Hospital Executive Summary Student Consultant, Radford Shiozaki & Rintaro Sato Community Partner, Dr
Our Lady of Lourdes Hospital Executive Summary Student Consultant, Radford Shiozaki & Rintaro Sato Community Partner, Dr. Abundio Palencia Jr. I. About the Organization Our Lady of Lourdes Hospital (OLLH) is a private, non-profit, 50-bed tertiary hospital in Daet, Camarines Norte, Philippines. The hospital provides essential medical services to the residents of the entire Bicol region. The late Dr. Abundio Palencia Sr. founded OLLH in 1965. Today, Dr. Palencia’s sons and daughter direct a majority of the hospital’s clinical and administrative roles. Official Vision To establish the hospital as one of the very best in the entire southern Luzon area that takes care of the sick as well as the healthy person in all of his dimension. Official Mission To serve the community in all of its health related needs. Recognizing that the person is the sum of all that he is, the hospital is geared towards the care and well being of all the parts of the person -- physical, emotional, mental, and spiritual. We will work to contribute to the attainment of a happy and healthy community. II. Improving Internal Communication and Information Management by Implementing a Framework for an EMR System One of the top priorities identified by the hospital staff was to create an easy way to retrieve medical data. Currently, patient charts are paper-based and stored in a room in the back of the hospital. The room is running out of physical space to store all the charts and some of the paper charts are getting damaged from mold and insects. The paper charts are also in danger of getting damaged from floods or fires. -

Brain Imaging Predictors and the International Study to Predict Optimized Treatment for Depression: Study Protocol for a Randomi
Grieve et al. Trials 2013, 14:224 http://www.trialsjournal.com/content/14/1/224 TRIALS STUDY PROTOCOL Open Access Brain imaging predictors and the international study to predict optimized treatment for depression: study protocol for a randomized controlled trial Stuart M Grieve1,2,3,4*, Mayuresh S Korgaonkar1,5, Amit Etkin6,7, Anthony Harris1,5, Stephen H Koslow8,9, Stephen Wisniewski7, Alan F Schatzberg6, Charles B Nemeroff8, Evian Gordon1,2 and Leanne M Williams1,5,6 Abstract Background: Approximately 50% of patients with major depressive disorder (MDD) do not respond optimally to antidepressant treatments. Given this is a large proportion of the patient population, pretreatment tests that predict which patients will respond to which types of treatment could save time, money and patient burden. Brain imaging offers a means to identify treatment predictors that are grounded in the neurobiology of the treatment and the pathophysiology of MDD. Methods/Design: The international Study to Predict Optimized Treatment in Depression is a multi-center, parallel model, randomized clinical trial with an embedded imaging sub-study to identify such predictors. We focus on brain circuits implicated in major depressive disorder and its treatment. In the full trial, depressed participants are randomized to receive escitalopram, sertraline or venlafaxine-XR (open-label). They are assessed using standardized multiple clinical, cognitive-emotional behavioral, electroencephalographic and genetic measures at baseline and at eight weeks post-treatment. Overall, 2,016 depressed participants (18 to 65 years old) will enter the study, of whom a target of 10% will be recruited into the brain imaging sub-study (approximately 67 participants in each treatment arm) and 67 controls.