Evaluation of DICOM Viewer Software for Workflow Integration in Clinical Trials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Large Sets of Image Data Capture, Databases, Image Processing, Storage, Visualization Karol Kozak
Management of large sets of image data Capture, Databases, Image Processing, Storage, Visualization Karol Kozak Download free books at Karol Kozak Management of large sets of image data Capture, Databases, Image Processing, Storage, Visualization Download free eBooks at bookboon.com 2 Management of large sets of image data: Capture, Databases, Image Processing, Storage, Visualization 1st edition © 2014 Karol Kozak & bookboon.com ISBN 978-87-403-0726-9 Download free eBooks at bookboon.com 3 Management of large sets of image data Contents Contents 1 Digital image 6 2 History of digital imaging 10 3 Amount of produced images – is it danger? 18 4 Digital image and privacy 20 5 Digital cameras 27 5.1 Methods of image capture 31 6 Image formats 33 7 Image Metadata – data about data 39 8 Interactive visualization (IV) 44 9 Basic of image processing 49 Download free eBooks at bookboon.com 4 Click on the ad to read more Management of large sets of image data Contents 10 Image Processing software 62 11 Image management and image databases 79 12 Operating system (os) and images 97 13 Graphics processing unit (GPU) 100 14 Storage and archive 101 15 Images in different disciplines 109 15.1 Microscopy 109 360° 15.2 Medical imaging 114 15.3 Astronomical images 117 15.4 Industrial imaging 360° 118 thinking. 16 Selection of best digital images 120 References: thinking. 124 360° thinking . 360° thinking. Discover the truth at www.deloitte.ca/careers Discover the truth at www.deloitte.ca/careers © Deloitte & Touche LLP and affiliated entities. Discover the truth at www.deloitte.ca/careers © Deloitte & Touche LLP and affiliated entities. -

Qualitative Comparison of Conventional and Oblique MRI for Detection of Herniated Spinal Discs
Qualitative Comparison of Conventional and Oblique MRI for Detection of Herniated Spinal Discs Doug Dean Final Project Presentation ENGN 2500: Medical Image Analysis May 16, 2011 Tuesday, May 17, 2011 Outline • Review of the problem presented in the paper: “A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen” (Authors: Shim JH, Park CK, Lee JH, et. al) • Approach to solve this problem • Data Acquisition • Analysis Methods • Results • Discussion/Conclusions Tuesday, May 17, 2011 Review of Problem • Difficult to identify herniated discs and spinal stenosis using conventional (2D) MRI techniques • These conventional methods result in patients condition being misdiagnosed. “Conventional MRI”: Images acquired along one of three anatomical planes Tuesday, May 17, 2011 3D reconstructive CT Axial, T2-weighted Image: Image shows that the Cervical Foramen is cervical foramina are directed at 45 degrees directed downward around with respect to coronal 10-15 degrees with plane. respect to axial plane Tuesday, May 17, 2011 Orientation of Images Conventional MRI: Sagittal Protocol Oblique MRI: Sagittal Protocol Tuesday, May 17, 2011 Timeline • Week 1 (4/11-4/16) • Work on developing MR imaging protocols and sequences • Recruit volunteers (~4-5 volunteers) • Week 2 (4/17-4/23) • Continue developing imaging sequences and begin data acquisition at the MRI facility • Assisted by Dr. Deoni • Week 3&4 (4/24-5/7) • Continue developing and testing sequence • 4/27/2011: Acquisition of first subject • Mid Project Presentation: Describe the imaging protocols, present data that had been acquired from previous week, describe what still needs to be done. -

MITK-Modelfit: a Generic Open-Source Framework for Model Fits and Their Exploration in Medical Imaging – Design, Implementatio
MITK-ModelFit: A generic open-source framework for model fits and their exploration in medical imaging – design, implementation and application on the example of DCE-MRI Charlotte Debus1-5,*,#, Ralf Floca5,6,*,#, Michael Ingrisch7, Ina Kompan5,6, Klaus Maier-Hein5,6,8, Amir Abdollahi1-5, and Marco Nolden6 1German Cancer Consortium (DKTK), Heidelberg, Germany 2Translational Radiation Oncology, German Cancer Research Center (DKFZ), Heidelberg, Germany 3Department of Radiation Oncology, Heidelberg Ion-Beam Therapy Center (HIT), Heidelberg University Hospital, Heidelberg, Germany 4National Center for Tumor Diseases (NCT), Heidelberg, Germany 5Heidelberg Institute of Radiation Oncology (HIRO), Germany 6Division of Medical Image Computing, German Cancer Research Center DKFZ, Germany 7Department of Radiology, University Hospital Munich, Ludwig-Maximilians-University Munich, Germany 8Section Pattern Recognition, Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany # Correspondence: Charlotte Debus, PhD Department of Translational Radiation Oncology Heidelberg Ion-Beam Therapy Center (HIT) Im Neuenheimer Feld 450 69120 Heidelberg, Germany Email: [email protected] Phone: +49 6221 6538281 Ralf Floca, PhD Division of Medical Image Computing German Cancer Research Center (DKFZ) Im Neuenheimer Feld 280 69120 Heidelberg, Germany Email: [email protected] Phone: + 49 6221 42 2560 * Shared first-authors 1 Abstract Background: Many medical imaging techniques utilize fitting approaches for quantitative parameter estimation and analysis. Common examples are pharmacokinetic modeling in dynamic contrast- enhanced (DCE) magnetic resonance imaging (MRI)/computed tomography (CT), apparent diffusion coefficient calculations and intravoxel incoherent motion modeling in diffusion-weighted MRI and Z- spectra analysis in chemical exchange saturation transfer MRI. Most available software tools are limited to a special purpose and do not allow for own developments and extensions. -

Survey of Databases Used in Image Processing and Their Applications
International Journal of Scientific & Engineering Research Volume 2, Issue 10, Oct-2011 1 ISSN 2229-5518 Survey of Databases Used in Image Processing and Their Applications Shubhpreet Kaur, Gagandeep Jindal Abstract- This paper gives review of Medical image database (MIDB) systems which have been developed in the past few years for research for medical fraternity and students. In this paper, I have surveyed all available medical image databases relevant for research and their use. Keywords: Image database, Medical Image Database System. —————————— —————————— 1. INTRODUCTION Measurement and recording techniques, such as electroencephalography, magnetoencephalography Medical imaging is the technique and process used to (MEG), Electrocardiography (EKG) and others, can create images of the human for clinical purposes be seen as forms of medical imaging. Image Analysis (medical procedures seeking to reveal, diagnose or is done to ensure database consistency and reliable examine disease) or medical science. As a discipline, image processing. it is part of biological imaging and incorporates radiology, nuclear medicine, investigative Open source software for medical image analysis radiological sciences, endoscopy, (medical) Several open source software packages are available thermography, medical photography and for performing analysis of medical images: microscopy. ImageJ 3D Slicer ITK Shubhpreet Kaur is currently pursuing masters degree OsiriX program in Computer Science and engineering in GemIdent Chandigarh Engineering College, Mohali, India. E-mail: MicroDicom [email protected] FreeSurfer Gagandeep Jindal is currently assistant processor in 1.1 Images used in Medical Research department Computer Science and Engineering in Here is the description of various modalities that are Chandigarh Engineering College, Mohali, India. E-mail: used for the purpose of research by medical and [email protected] engineering students as well as doctors. -
PDF File, 212 KB
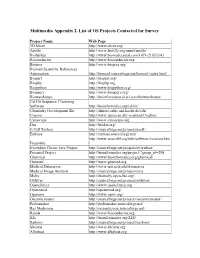
Multimedia Appendix 2. List of OS Projects Contacted for Survey Project Name Web Page 3D Slicer http://www.slicer.org/ Apollo http://www.fruitfly.org/annot/apollo/ Biobuilder http://www.biomedcentral.com/1471-2105/5/43 Bioconductor http://www.bioconductor.org Biojava http://www.biojava.org/ Biomail Scientific References Automation http://biomail.sourceforge.net/biomail/index.html Bioperl http://bioperl.org/ Biophp http://biophp.org Biopython http://www.biopython.org/ Bioquery http://www.bioquery.org/ Biowarehouse http://bioinformatics.ai.sri.com/biowarehouse/ Cd-Hit Sequence Clustering Software http://bioinformatics.org/cd-hit/ Chemistry Development Kit http://almost.cubic.uni-koeln.de/cdk/ Coasim http://www.daimi.au.dk/~mailund/CoaSim/ Cytoscape http://www.cytoscape.org Das http://biodas.org/ E-Cell System http://sourceforge.net/projects/ecell/ Emboss http://emboss.sourceforge.net/ http://www.ensembl.org/info/software/versions.htm Ensemble l Eviewbox Dicom Java Project http://sourceforge.net/projects/eviewbox/ Freemed Project http://bioinformatics.org/project/?group_id=298 Ghemical http://www.bioinformatics.org/ghemical/ Gnumed http://www.gnumed.org Medical Dataserver http://www.mii.ucla.edu/dataserver Medical Image Analysis http://sourceforge.net/projects/mia Moby http://biomoby.open-bio.org/ Olduvai http://sourceforge.net/projects/olduvai/ Openclinica http://www.openclinica.org Openemed http://openemed.org/ Openemr http://www.oemr.org/ Oscarmcmaster http://sourceforge.net/projects/oscarmcmaster/ Probemaker http://probemaker.sourceforge.net/ -
Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Version 1.0 2018-08-30
NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the NNOSEasal Cavity and Paranasal Sinuses NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Version 1.0 2018-08-30 NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation Version 1.0 of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses Project Number 1000043433 Project Title NOSE Pilot Study Document Reference Investigating the Practical Use of Computational Date 2018-08-30 Title Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses Document Name NOSE_PS_FINAL_v1 Version Draft draft final Restrictions public internal restricted : Distribution Steirische Forschungsförderung Authors Koch Walter, Koch Gerda, Vitiello Massimo, Ortiz Ramiro, Stockklauser Jutta, Benda Odo Abstract The NOSE Pilot study evaluated the technical and scientific environment required for establishing a service portfolio that includes CFD simulation and 3D visualization services for ENT specialists. For this purpose the state-of-the-art of these technologies and their use for upper airways diagnostics were analysed. Keywords Rhinology, Computational Fluid Dynamics, 3D Visualization, Clinical Pathways, Service Center, Knowledge Base Document Revisions Version Date Author(s) Description of Change 1.0 2018-08-30 Koch Walter, Koch Final Version Gerda, Vitiello Massimo, Ortiz NOSERamiro, Stockklauser Jutta, Benda Odo 2018-08-30 Seite 3 / 82 Copyright © AIT ForschungsgesmbH NOSE_PS_FINAL_v1 NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation Version 1.0 of Airflow in the Nasal Cavity and Paranasal Sinuses Table of Contents Acknowledgments .................................................................................. -

Whole Body Computed Tomography with Advanced Imaging Techniques: a Research Tool for Measuring Body Composition in Dogs
Hindawi Publishing Corporation Journal of Veterinary Medicine Volume 2013, Article ID 610654, 6 pages http://dx.doi.org/10.1155/2013/610654 Research Article Whole Body Computed Tomography with Advanced Imaging Techniques: A Research Tool for Measuring Body Composition in Dogs Dharma Purushothaman,1 Barbara A. Vanselow,2 Shu-Biao Wu,1 Sarah Butler,3 and Wendy Yvonne Brown1 1 School of Environmental and Rural Science, Department of Animal Science, University of New England, Armidale, NSW 2351, Australia 2 NSW Department of Primary Industries, Beef Industry Centre, University of New England, Armidale, NSW 2351, Australia 3 North Hill Vet Clinic, Armidale, NSW 2350, Australia Correspondence should be addressed to Wendy Yvonne Brown; [email protected] Received 6 May 2013; Revised 14 September 2013; Accepted 17 September 2013 Academic Editor: Juan G. Chediack Copyright © 2013 Dharma Purushothaman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The use of computed tomography (CT) to evaluate obesity in canines is limited. Traditional CT image analysis is cumbersome and uses prediction equations that require manual calculations. In order to overcome this, our study investigated the use of advanced image analysis software programs to determine body composition in dogs with an application to canine obesity research. Beagles and greyhounds were chosen for their differences in morphology and propensity to obesity. Whole body CT scans with regular intervals were performed on six beagles and six greyhounds that were subjected to a 28-day weight-gain protocol. -

Impacting the Bioscience Progress by Backporting Software for Bio-Linux
Impacting the bioscience progress by backporting software for Bio-Linux Sasa Paporovic [email protected] v0.9 What is Bio-Linux and what is it good for - also its drawbacks: If someone says to use or to have a Linux this is correct as like it is imprecise. It does not exist a Linux as full functional operating system by itself. What was originally meant by the term Linux was the operating system core[1]. The so called kernel, or in a case of a Linux operating system the Linux kernel. It is originally designed and programmed by Linus Torvalds, who is also today the developer in chef or to say it with his words, he is the “alpha-male” of all developers[2]. Anyway, what we have today are Distributions[3]. It has become common to call them simply “a Linux”. This means that there are organizations out there, mostly private, some funded and some other commercial, which gather all what is needed to design around the Linux kernel a full functional operating system. This targets mostly Software, but also web and service infrastructure. Some of them have a history that is nearly as long as the Linux kernel is alive, like Debian. Some others are younger like Ubuntu and some more others are very young, like Bio-Linux[4]. The last Linux, the Bio-Linux, especially its latest version Bio-Linux 7 we are focusing here[5]. In year 2006 Bio-Linux with the work of Tim Booth[42] and team gives its rising[6] and provide an operating system that was and still specialized in providing a bioinformatic specific software environment for the working needs in this corner of bioscience. -

Kitware Source Issue 21
SOFTWARE DEVELOPER’S QUARTERLY Issue 21 • April 2012 Editor’s Note ........................................................................... 1 PARAVIEW 3.14 RELEASED In late February, Kitware and the ParaView team released Recent Releases ..................................................................... 1 ParaView 3.14. This release features usability enhancements, improvements to the Plugin framework, new panels, and ParaView in Immersive Environments .................................. 3 more than 100 other resolved issues. Teaching Open Source .......................................................... 4 ParaView 3.14 features a redesigned Find Data dialog and Color Editor. The updated Find Data dialog makes it possible ParaView Query Selection Framework................................. 7 to use complex queries to select elements, including combin- ing multiple test cases with Boolean operations. The Color Ginkgo CADx: Open Source DICOM CADx Environment .... 8 Editor, used to edit lookup tables or color tables for scalar mapping, now enables independent editing of the color and Code Review, Topic Branches and VTK ................................. 9 opacity functions. Video Analysis on Business Intelligence, ParaView’s charting capabilities have been extended with a Studies in Computational Intelligence ............................... 11 new scatter plot matrix view and the ability to visualize mul- tiple dimensions of data in one compact form. This improved The Visible Patient .............................................................. -

A Case Study from the Openmrs Open-Source Radiology Information System
Journal of Digital Imaging (2018) 31:361–370 https://doi.org/10.1007/s10278-018-0088-5 A Platform for Innovation and Standards Evaluation: a Case Study from the OpenMRS Open-Source Radiology Information System Judy W. Gichoya1 & Marc Kohli2 & Larry Ivange3 & Teri S. Schmidt4 & Saptarshi Purkayastha5 Published online: 10 May 2018 # The Author(s) 2018 Abstract Open-source development can provide a platform for innovation by seeking feedback from community members as well as providing tools and infrastructure to test new standards. Vendors of proprietary systems may delay adoption of new standards until there are sufficient incentives such as legal mandates or financial incentives to encourage/mandate adoption. Moreover, open-source systems in healthcare have been widely adopted in low- and middle-income countries and can be used to bridge gaps that exist in global health radiology. Since 2011, the authors, along with a community of open-source contributors, have worked on developing an open-source radiology information system (RIS) across two communities—OpenMRS and LibreHealth. The main purpose of the RIS is to implement core radiology workflows, on which others can build and test new radiology standards. This work has resulted in three major releases of the system, with current architectural changes driven by changing technology, development of new standards in health and imaging informatics, and changing user needs. At their core, both these communities are focused on building general-purpose EHR systems, but based on user contributions from the fringes, we have been able to create an innovative system that has been used by hospitals and clinics in four different countries. -

Transforming the Medical Imaging Workflow
Transforming the Medical Imaging Workflow: How Mac systems and open source software combine to make full-featured diagnostic imaging solutions affordable for today’s radiologist. By Roger Katen, M.D. San Francisco, CA White Paper 2 Transforming the Medical Imaging Workflow Contents Page 3 Executive Summary Page 5 The New Realities of Medical Imaging The Digital Data Explosion The Rise of Imaging Workstations A Cost Conundrum Page 8 A Compelling Alternative: The Mac and OsiriX Workstation OsiriX: Full-Featured, Open Source Mac OS X and Mac Systems A New Solution for a New Reality Page 10 Mac Systems and OsiriX in the Radiology Workflow Image Generation Equipment Picture Archiving and Communications Systems (PACS) Creating a PACS Archive with OsiriX Database Sharing and Autorouting Storage System Page 16 Building an Imaging Workstation Environment with OsiriX Working with Large Datasets Supporting Medical-Quality Displays Requirements for High-Performance Processors Accelerating Image Retrieval Page 19 Enabling the Imaging Workflow Options for Advanced Visualization Starting and Sending a New DICOM Series to PACS Emailing and Printing Images Integrating with Hospital and Radiology Information Systems Exploring Alternatives to Windows RIS Systems Page 24 Collaborating Beyond the Office with OsiriX Virtual Private Networks Remote Visualization via Apple Remote Desktop Citrix/Windows Terminal Services Teleradiology Collaboration iChat and iChat Theater Page 28 Conclusion White Paper 3 Transforming the Medical Imaging Workflow Executive Summary Today more than ever, the healthcare industry is reaping the benefits of advances in diagnostic medical imaging. Dramatic breakthroughs in higher-resolution CT, MRI, ultrasound, and interventional technologies have enabled healthcare providers to deliver more informed diagnoses, pursue more effective treatments, collaborate more easily with colleagues, and communicate more clearly with patients. -
FOSDEM 2013 Schedule
FOSDEM 2013 - Saturday 2013-02-02 (1/9) Janson K.1.105 Ferrer Chavanne Lameere H.1301 H.1302 H.1308 10:30 Welcome to FOSDEM 2013 10:45 11:00 How we made the Jenkins QEMU USB status report 2012 Rockbuild The neat guide to Fedora RPM LinuxonAndroid and SlapOS on Wayland for Application Developers community Packaging Android 11:15 11:30 CRIU: Checkpoint and Restore (mostly) In Userspace 11:45 Ubuntu Online Accounts for application developers 12:00 The Devil is in the Details Vtrill: Rbridges for Virtual PTXdist Building RPM packages from Git Emdedded distro shootout: buildroot Networking repositories with git-buildpackage vs. Debian Better software through user 12:15 research 12:30 Bringing Xen to CentOS-6 Sketching interactions 12:45 13:00 The Open Observatory of Network Porting Fedora to 64-bit ARM Coding Goûter A brief tutorial on Xen's advanced Guacamayo -- Building Multimedia Package management and creation ARM v7 State of the Body ↴ Interference systems security features Appliance with Yocto ↴ in Gentoo Linux ↴ 13:15 Spoiling and Counter-spoiling 13:30 oVirt Live Storage Migration - Under Modern CMake ↴ the Hood ↴ ZONE: towards a better news feed ↴ 13:45 FOSDEM 2013 - Saturday 2013-02-02 (2/9) H.1309 H.2213 H.2214 AW1.120 AW1.121 AW1.125 AW1.126 Guillissen 10:30 10:45 11:00 Metaphor and BDD XMPP 101 Storytelling FLOSS Welcome and Introduction The room open() process Scripting Apache OpenOffice: Welcome to the Perl d… Introductory Nutshell Programs Inheritance versus Roles (Writer, Calc, Impress) 11:15 Signal/Collect: Processing Large