Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Successful Treatment of Rifampicin Resistant Case of Leprosy by WHO Recommended Ofloxacin and Minocycline Regimen
Lepr Rev (2019) 90, 456–459 CASE REPORT Successful treatment of rifampicin resistant case of leprosy by WHO recommended ofloxacin and minocycline regimen MALLIKA LAVANIAa, JOYDEEPA DARLONGb, ABHISHEK REDDYb, MADHVI AHUJAa, ITU SINGHa, R.P. TURANKARa & U. SENGUPTAa aStanley Browne Laboratory, The Leprosy Mission Community Hospital, Nand Nagri, New Delhi 110093, India bThe Leprosy Mission Hospital, Purulia, West Bengal 723101, India Accepted for publication 15 July 2019 Summary A 25-year-old male treated for leprosy at the age of 15, with MDT, visited TLM Purulia Hospital in July 2017. He was provisionally diagnosed as a lepromatous relapse with ENL reaction. A biopsy was done to test for drug resistance. Drug resistance testing showed resistance to rifampicin. Second line drug regimen recommended by WHO for rifampicin-resistance was started. Within 6 months of taking medication the clinical signs and symptoms improved rapidly and the BI dropped by 1·66 log within 6 months. This case highlights the need for investigations in cases of relapse and the efficacy of WHO recommended second line drug regimen treatment in rifampicin-resistant leprosy cases. Keywords: leprosy, rifampicin-resistance, second-line treatment Introduction Leprosy, also known as Hansen’s disease (HD), is a chronic infectious disease caused by the bacterium Mycobacterium leprae or Mycobacterium lepromatosis.1,2 Leprosy is curable with administration of a rifampicin, clofazimine and dapsone known as multidrug therapy (MDT). Since the introduction of MDT in 1983 the prevalence -

Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications
International Journal of Molecular Sciences Review Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications Daniel Fernández-Villa 1, Maria Rosa Aguilar 1,2 and Luis Rojo 1,2,* 1 Instituto de Ciencia y Tecnología de Polímeros, Consejo Superior de Investigaciones Científicas, CSIC, 28006 Madrid, Spain; [email protected] (D.F.-V.); [email protected] (M.R.A.) 2 Consorcio Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-915-622-900 Received: 18 September 2019; Accepted: 7 October 2019; Published: 9 October 2019 Abstract: Bacterial, protozoan and other microbial infections share an accelerated metabolic rate. In order to ensure a proper functioning of cell replication and proteins and nucleic acids synthesis processes, folate metabolism rate is also increased in these cases. For this reason, folic acid antagonists have been used since their discovery to treat different kinds of microbial infections, taking advantage of this metabolic difference when compared with human cells. However, resistances to these compounds have emerged since then and only combined therapies are currently used in clinic. In addition, some of these compounds have been found to have an immunomodulatory behavior that allows clinicians using them as anti-inflammatory or immunosuppressive drugs. Therefore, the aim of this review is to provide an updated state-of-the-art on the use of antifolates as antibacterial and immunomodulating agents in the clinical setting, as well as to present their action mechanisms and currently investigated biomedical applications. Keywords: folic acid antagonists; antifolates; antibiotics; antibacterials; immunomodulation; sulfonamides; antimalarial 1. -

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

RIFAMPICIN Productinformation Sigma Prod
RIFAMPICIN ProductInformation Sigma Prod. No. R3501 CH3 CH3 CAS NUMBER: 13292-46-1 HO SYNONYMS: Tubocin; Sinerdol; Rimactan; L-5103; Dione-21 Acetate; Archidyn; Arficin; 3-(4- CH3 O O OH O Methylpiperazinyliminomethyl)-rifamycin SV; NSC 113926; C OH OH CH 1 2 3 H C Rifampin ; Rifaldazine; Rifamycin AMP H3C 3 O NH H3C PHYSICAL PROPERTIES: CH3 N CH N Appearance: Orange-brown to red-brown powder.3 O OH N Molecular formula: C43H58N4O12 O Molecular weight: 823.0 O CH3 CH3 EmM (max absorbance, phosphate buffer, pH 7.38): 33.20 (237 nm); 32.10 (255 nm); 27.00 (334 nm); 15.40 (475 nm)2,4 pKa (in water):1.7 (4-hydroxyl group), 7.9 (4-piperazine nitrogen); in methylcellosolve-water (4:1): 3.6 (4- hydroxyl group), 6.7 (3-piperazine nitrogen)4 pI (in water): 4.84 25° 4 Optical rotation: [α]D =+10.6° (c=0.5% in CDCl3) Melting point: 183-188°C (dec.)2,4 METHOD OF PREPARATION: Methods of preparation have been reported.4,5 The NMR, UV, IR, Mass spectra, Thin-Layer chromatography and HPLC methods of detection have been reported.4,5,6 A colorimetric test for identification was reported.4 STABILITY / STORAGE: Rifampicin (Rif) should be stable for at least two years when stored desiccated at -20°C and protected from light.3 Rif is stable as a solid at temperatures up to 70EC.4 SOLUBILITY / SOLUTION STABILITY: Rif is soluble in dimethylsulfoxide (~100mg/mL), dimethylformamide, methanol (16 mg/ml, 25EC), chloroform (349 mg/ml, 25°C), ethyl acetate (108 mg/ml, 25°C), and acetone (14 mg/ml, 25°C).4,6,7,8,9 Rif is slightly soluble in water at 25°C: 2.5 mg/ml, pH 7.3; 1.3 mg/ml, pH 4.3; and in 95% ethanol (∼10 mg/mL).4 Rif is soluble at 37°C: in 0.1 N HCl, 200 mg/ml and in phosphate buffer pH 7.4, 9.9 mg/ml.4 R3501 Page 1 of 4 03/28/97 - ARO RIFAMPICIN Sigma Prod. -

Clofazimine As a Treatment for Multidrug-Resistant Tuberculosis: a Review
Scientia Pharmaceutica Review Clofazimine as a Treatment for Multidrug-Resistant Tuberculosis: A Review Rhea Veda Nugraha 1 , Vycke Yunivita 2 , Prayudi Santoso 3, Rob E. Aarnoutse 4 and Rovina Ruslami 2,* 1 Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung 40161, Indonesia; [email protected] 2 Division of Pharmacology and Therapy, Department of Biomedical Sciences, Faculty of Medicine, Universitas Padjadjaran, Bandung 40161, Indonesia; [email protected] 3 Department of Internal Medicine, Faculty of Medicine, Universitas Padjadjaran—Hasan Sadikin Hospital, Bandung 40161, Indonesia; [email protected] 4 Department of Pharmacy, Radboud University Medical Center, Radboud Institute for Health Sciences, 6255HB Nijmegen, The Netherlands; [email protected] * Correspondence: [email protected] Abstract: Multidrug-resistant tuberculosis (MDR-TB) is an infectious disease caused by Mycobac- terium tuberculosis which is resistant to at least isoniazid and rifampicin. This disease is a worldwide threat and complicates the control of tuberculosis (TB). Long treatment duration, a combination of several drugs, and the adverse effects of these drugs are the factors that play a role in the poor outcomes of MDR-TB patients. There have been many studies with repurposed drugs to improve MDR-TB outcomes, including clofazimine. Clofazimine recently moved from group 5 to group B of drugs that are used to treat MDR-TB. This drug belongs to the riminophenazine class, which has lipophilic characteristics and was previously discovered to treat TB and approved for leprosy. This review discusses the role of clofazimine as a treatment component in patients with MDR-TB, and Citation: Nugraha, R.V.; Yunivita, V.; the drug’s properties. -

Analysis of Mutations Leading to Para-Aminosalicylic Acid Resistance in Mycobacterium Tuberculosis
www.nature.com/scientificreports OPEN Analysis of mutations leading to para-aminosalicylic acid resistance in Mycobacterium tuberculosis Received: 9 April 2019 Bharati Pandey1, Sonam Grover2, Jagdeep Kaur1 & Abhinav Grover3 Accepted: 31 July 2019 Thymidylate synthase A (ThyA) is the key enzyme involved in the folate pathway in Mycobacterium Published: xx xx xxxx tuberculosis. Mutation of key residues of ThyA enzyme which are involved in interaction with substrate 2′-deoxyuridine-5′-monophosphate (dUMP), cofactor 5,10-methylenetetrahydrofolate (MTHF), and catalytic site have caused para-aminosalicylic acid (PAS) resistance in TB patients. Focusing on R127L, L143P, C146R, L172P, A182P, and V261G mutations, including wild-type, we performed long molecular dynamics (MD) simulations in explicit solvent to investigate the molecular principles underlying PAS resistance due to missense mutations. We found that these mutations lead to (i) extensive changes in the dUMP and MTHF binding sites, (ii) weak interaction of ThyA enzyme with dUMP and MTHF by inducing conformational changes in the structure, (iii) loss of the hydrogen bond and other atomic interactions and (iv) enhanced movement of protein atoms indicated by principal component analysis (PCA). In this study, MD simulations framework has provided considerable insight into mutation induced conformational changes in the ThyA enzyme of Mycobacterium. Antimicrobial resistance (AMR) threatens the efective treatment of tuberculosis (TB) caused by the bacteria Mycobacterium tuberculosis (Mtb) and has become a serious threat to global public health1. In 2017, there were reports of 5,58000 new TB cases with resistance to rifampicin (frst line drug), of which 82% have developed multidrug-resistant tuberculosis (MDR-TB)2. AMR has been reported to be one of the top health threats globally, so there is an urgent need to proactively address the problem by identifying new drug targets and understanding the drug resistance mechanism3,4. -

Leprosy – Eliminated and Forgotten: a Case Report Shiva Raj K.C.1,5* , Geetika K.C.1, Purnima Gyawali2, Manisha Singh3 and Milesh Jung Sijapati4
K.C. et al. Journal of Medical Case Reports (2019) 13:276 https://doi.org/10.1186/s13256-019-2198-1 CASE REPORT Open Access Leprosy – eliminated and forgotten: a case report Shiva Raj K.C.1,5* , Geetika K.C.1, Purnima Gyawali2, Manisha Singh3 and Milesh Jung Sijapati4 Abstract Background: Leprosy is a disease that was declared eliminated in 2010 from Nepal; however, new cases are diagnosed every year. The difficulty arises when the presentation of the patient is unusual. Case presentation: In this case report we present a case of a 22-year-old Tamang man, from the Terai region of Nepal, with a clinical presentation of fever, malaise, and arthralgia for the past 2 weeks with hepatosplenomegaly and bilateral cervical, axillary, and inguinal lymphadenopathy. Features of chronic inflammation with elevated erythrocyte sedimentation rate of 90 mm/hour and liver enzymes were noted. With no specific investigative findings, a diagnosis of Still’s disease was made and he was given prednisolone. On tapering the medication, after 2 weeks, the lymphadenopathy and fever reappeared. On biopsy of a lymph node, diagnosis of possible tuberculosis was made. On that basis anti-tuberculosis treatment category I was started. During his hospital stay, our patient developed nodular skin rashes on his shoulder, back, and face. The biopsy of a skin lesion showed erythema nodosum leprosum and he was diagnosed as having lepromatous leprosy with erythema nodosum leprosum; he was treated with anti-leprosy medication. Conclusion: An unusual presentations of leprosy may delay its prompt diagnosis and treatment; thus, increasing morbidity and mortality. -

Lepromatous Leprosy Simulating Sweet Syndrome
ISSN: 2469-5750 Zemmez et al. J Dermatol Res Ther 2018, 4:056 DOI: 10.23937/2469-5750/1510056 Volume 4 | Issue 1 Journal of Open Access Dermatology Research and Therapy CASE REPORT Lepromatous Leprosy Simulating Sweet Syndrome Youssef Zemmez1*, Ahmed Bouhamidi1, Salwa Belhabib2, Rachid Frikh1, Mohamed Boui1 1 and Naoufal Hjira Check for updates 1Department of Dermatology-Venereology, Mohammed V Military Training Hospital, Rabat, Morocco 2Department of Pathological Anatomy, Mohammed V Military Training Hospital, Rabat, Morocco *Corresponding author: Youssef Zemmez, Department of Dermatology-Venereology, Mohammed V Military Training Hospital, Rabat, Morocco, Tel: 0658150805, E-mail: [email protected] Abstract Leprosy or Hansen's disease is an infection by Mycobac- terium leprae (M. leprae), whose prevalence has consid- erably decreased since the application of the new anti-lep- rosy strategies advocated since 1982 by the World Health Organization (WHO). However, in the endemic countries several cases of leprosy are reported annually. We report a clinical case of lepromatous leprosy revealed by dissemi- nated maculopapular lesions simulating a Sweet syndrome highlighting the importance of knowing how to evoke this diagnosis in patients from endemic areas. Keywords Lepromatous leprosy, Rash, Sweet syndrome Introduction Lepromatous leprosy is generally manifested by Figure 1: a) Maculopapular erythematous lesions of the non-inflammatory lesions, hypochromic macules, and face; b) Papulonodular erythematous lesions in forearms progressive erythematous papulo-nodules. We report and hands. an observation of lepromatous leprosy in a 62-year-old patient from rural Morocco who was diagnosed with abdomen (Figure 2a and Figure 2b). The neurological maculopapular lesions. examination showed hypoesthesia in gloves and socks, with bilateral hypertrophy of the ulnar nerve. -
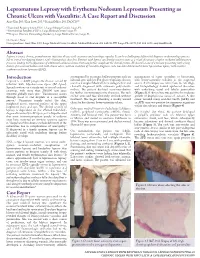
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Lepromatous Leprosy Masquerading As Rhinophyma
International Journal of Otorhinolaryngology and Head and Neck Surgery Krishna S et al. Int J Otorhinolaryngol Head Neck Surg. 2015 Jul;1(1):34-36 http://www.ijorl.com pISSN 2454-5929 | eISSN 2454-5937 DOI: http://dx.doi.org/10.18203/issn.2454-5929.ijohns20150585 Case Report Lepromatous leprosy masquerading as rhinophyma 1 1 1 2 Sowmyashree Krishna *, Malcolm Pinto , Manjunath Mala Shenoy , Mahesh SG 1 Department of Dermatology, Yenepoya Medical College, Yenepoya University, Mangalore, Karnataka, India 2Department of Otolaryngology-Head and Neck Surgery, A.J. Institute of Medical Sciences and Research Center, Mangalore, Karnataka, India Received: 26 May 2015 Accepted: 24 June 2015 *Correspondence: Dr. Sowmyashree Krishna, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Leprosy a major global health problem, especially in the developing world, is an infectious disease caused by Mycobacterium leprae. Leprosy has a predilection to with cooler areas of the body. Lepromatous leprosy presents with varied manifestations like nodules, cervical lymphadenitis, hyperpigmented patches and other presentations which can mimic various other diseases and pose a diagnostic challenge in endemic areas. We report a case presenting with nodular infiltration of the nose mimicking rhinophyma who presented with faint reddish swelling over the nose which progressed to nodular infiltration. There was bilateral symmetrical thickening of nerves following which diagnosis was confirmed by slit skin smear and the patient was started on multibacillary multidrug therapy. -

PROCUR Why Procur Has Been Prescribed for You
Consumer Medicine Information Ask your doctor if you have any questions about PROCUR why Procur has been prescribed for you. Cyproterone acetate 50 mg and 100 mg tablets This medicine is available only with a doctor's prescription. What is in this leaflet Before you take Procur Please read this leaflet carefully before you start taking Procur When you must not take it This leaflet answers some common questions about Procur. It does not contain all the available Do not take Procur if you have an allergy to: information. It does not take the place of talking • any medicine containing cyproterone acetate to your doctor or pharmacist. • any of the ingredients listed at the end of this leaflet All medicines have risks and benefits. Your doctor has weighed the risks of you taking Procur against Some of the symptoms of an allergic reaction may the benefits they expect it will have for you. include: • difficulty in breathing or wheezing If you have any concerns about taking this • shortness of breath medicine, ask your doctor or pharmacist. • swelling of the face, tongue, lips, or other parts of the body Keep this leaflet with the medicine. You may • hives on the skin, rash, or itching need to read it again. Do not take Procur if: What Procur is used for • you are allergic to cyproterone acetate or any other ingredient listed at the end of this leaflet Procur tablets contain the active ingredient • you are pregnant cyproterone acetate. Cyproterone acetate is an • you are breastfeeding antiandrogen. It works by blocking the actions of • you suffer from liver diseases (including sex hormones (androgens) that are produced previous or existing liver tumours, Dubin- mainly in men but also, to a lesser extent in Johnson syndrome or Rotor syndrome) women. -

Toman's Tuberculosis Case Detection, Treatment, and Monitoring
TOMAN’S TUBERCULOSIS TOMAN’S TUBERCULOSIS CASE DETECTION, TREATMENT, AND MONITORING The second edition of this practical, authoritative reference book provides a rational basis for the diagnosis and management of tuberculosis. Written by a number of experts in the field, it remains faithful to Kurt Toman’s original question-and-answer format, with subject matter grouped under the three headings Case detection, Treatment, and Monitoring. It is a testament to the enduring nature of the first edition that so much CASE DETECTION, TREA material has been retained unchanged. At the same time, the new edition has had not only to address the huge resurgence of tuber- culosis, the emergence of multidrug-resistant bacilli, and the special needs of HIV-infected individuals with tuberculosis, but also to encompass significant scientific advances. These changes in the profile of the disease and in approaches to management have inevitably prompted many new questions and answers and given a different complexion to others. Toman’s Tuberculosis remains essential reading for all who need to AND MONITORING TMENT, QUESTIONS learn more about every aspect of tuberculosis – case-finding, manage- ment, and effective control strategies. It provides invaluable support AND to anyone in the front line of the battle against this disease, from ANSWERS programme managers to policy-makers and from medical personnel to volunteer health workers. SECOND EDITION ISBN 92 4 154603 4 WORLD HEALTH ORGANIZATION WHO GENEVA Toman’s Tuberculosis Case detection, treatment, and monitoring – questions and answers SECOND EDITION Edited by T. Frieden WORLD HEALTH ORGANIZATION GENEVA 2004 WHO Library Cataloguing-in-Publication Data Toman’s tuberculosis case detection, treatment, and monitoring : questions and answers / edited by T.