Pharmacological Interaction of Lopinavir
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RIFAMPICIN Productinformation Sigma Prod
RIFAMPICIN ProductInformation Sigma Prod. No. R3501 CH3 CH3 CAS NUMBER: 13292-46-1 HO SYNONYMS: Tubocin; Sinerdol; Rimactan; L-5103; Dione-21 Acetate; Archidyn; Arficin; 3-(4- CH3 O O OH O Methylpiperazinyliminomethyl)-rifamycin SV; NSC 113926; C OH OH CH 1 2 3 H C Rifampin ; Rifaldazine; Rifamycin AMP H3C 3 O NH H3C PHYSICAL PROPERTIES: CH3 N CH N Appearance: Orange-brown to red-brown powder.3 O OH N Molecular formula: C43H58N4O12 O Molecular weight: 823.0 O CH3 CH3 EmM (max absorbance, phosphate buffer, pH 7.38): 33.20 (237 nm); 32.10 (255 nm); 27.00 (334 nm); 15.40 (475 nm)2,4 pKa (in water):1.7 (4-hydroxyl group), 7.9 (4-piperazine nitrogen); in methylcellosolve-water (4:1): 3.6 (4- hydroxyl group), 6.7 (3-piperazine nitrogen)4 pI (in water): 4.84 25° 4 Optical rotation: [α]D =+10.6° (c=0.5% in CDCl3) Melting point: 183-188°C (dec.)2,4 METHOD OF PREPARATION: Methods of preparation have been reported.4,5 The NMR, UV, IR, Mass spectra, Thin-Layer chromatography and HPLC methods of detection have been reported.4,5,6 A colorimetric test for identification was reported.4 STABILITY / STORAGE: Rifampicin (Rif) should be stable for at least two years when stored desiccated at -20°C and protected from light.3 Rif is stable as a solid at temperatures up to 70EC.4 SOLUBILITY / SOLUTION STABILITY: Rif is soluble in dimethylsulfoxide (~100mg/mL), dimethylformamide, methanol (16 mg/ml, 25EC), chloroform (349 mg/ml, 25°C), ethyl acetate (108 mg/ml, 25°C), and acetone (14 mg/ml, 25°C).4,6,7,8,9 Rif is slightly soluble in water at 25°C: 2.5 mg/ml, pH 7.3; 1.3 mg/ml, pH 4.3; and in 95% ethanol (∼10 mg/mL).4 Rif is soluble at 37°C: in 0.1 N HCl, 200 mg/ml and in phosphate buffer pH 7.4, 9.9 mg/ml.4 R3501 Page 1 of 4 03/28/97 - ARO RIFAMPICIN Sigma Prod. -

PROCUR Why Procur Has Been Prescribed for You
Consumer Medicine Information Ask your doctor if you have any questions about PROCUR why Procur has been prescribed for you. Cyproterone acetate 50 mg and 100 mg tablets This medicine is available only with a doctor's prescription. What is in this leaflet Before you take Procur Please read this leaflet carefully before you start taking Procur When you must not take it This leaflet answers some common questions about Procur. It does not contain all the available Do not take Procur if you have an allergy to: information. It does not take the place of talking • any medicine containing cyproterone acetate to your doctor or pharmacist. • any of the ingredients listed at the end of this leaflet All medicines have risks and benefits. Your doctor has weighed the risks of you taking Procur against Some of the symptoms of an allergic reaction may the benefits they expect it will have for you. include: • difficulty in breathing or wheezing If you have any concerns about taking this • shortness of breath medicine, ask your doctor or pharmacist. • swelling of the face, tongue, lips, or other parts of the body Keep this leaflet with the medicine. You may • hives on the skin, rash, or itching need to read it again. Do not take Procur if: What Procur is used for • you are allergic to cyproterone acetate or any other ingredient listed at the end of this leaflet Procur tablets contain the active ingredient • you are pregnant cyproterone acetate. Cyproterone acetate is an • you are breastfeeding antiandrogen. It works by blocking the actions of • you suffer from liver diseases (including sex hormones (androgens) that are produced previous or existing liver tumours, Dubin- mainly in men but also, to a lesser extent in Johnson syndrome or Rotor syndrome) women. -

Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
pharmaceutics Review Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Luíse L. Chaves 1,2,*, Yuri Patriota 2, José L. Soares-Sobrinho 2 , Alexandre C. C. Vieira 1,3, Sofia A. Costa Lima 1,4 and Salette Reis 1,* 1 Laboratório Associado para a Química Verde, Rede de Química e Tecnologia, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, 4050-313 Porto, Portugal; [email protected] (A.C.C.V.); slima@ff.up.pt (S.A.C.L.) 2 Núcleo de Controle de Qualidade de Medicamentos e Correlatos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil; [email protected] (Y.P.); [email protected] (J.L.S.-S.) 3 Laboratório de Tecnologia dos Medicamentos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil 4 Cooperativa de Ensino Superior Politécnico e Universitário, Instituto Universitário de Ciências da Saúde, 4585-116 Gandra, Portugal * Correspondence: [email protected] (L.L.C.); shreis@ff.up.pt (S.R.) Received: 30 October 2020; Accepted: 4 December 2020; Published: 11 December 2020 Abstract: Leprosy disease remains an important public health issue as it is still endemic in several countries. Mycobacterium leprae, the causative agent of leprosy, presents tropism for cells of the reticuloendothelial and peripheral nervous system. Current multidrug therapy consists of clofazimine, dapsone and rifampicin. Despite significant improvements in leprosy treatment, in most programs, successful completion of the therapy is still sub-optimal. Drug resistance has emerged in some countries. This review discusses the status of leprosy disease worldwide, providing information regarding infectious agents, clinical manifestations, diagnosis, actual treatment and future perspectives and strategies on targets for an efficient targeted delivery therapy. -

Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis
Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Centers for Disease Control and Prevention Office of Infectious Diseases National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination June 2013 This document is accessible online at http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Suggested citation: CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online]. 2013. Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Table of Contents Introduction 1 Methodology for Preparation of these Guidelines 2 The Role of Rifamycins in Tuberculosis Treatment 4 Managing Drug Interactions with Antivirals and Rifampin 5 Managing Drug Interactions with Antivirals and Rifabutin 9 Treatment of Latent TB Infection with Rifampin or Rifapentine 10 Treating Pregnant Women with Tuberculosis and HIV Co-infection 10 Treating Children with HIV-associated Tuberculosis 12 Co-treatment of Multidrug-resistant Tuberculosis and HIV 14 Limitations of these Guidelines 14 HIV-TB Drug Interaction Guideline Development Group 15 References 17 Table 1a. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in adults 21 Table 1b. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in children 22 Table 2a. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in adults 23 Table 2b. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in children 25 Table 3. Recommendations for co-administering antiretroviral drugs with RIFABUTIN in adults 26 ii Introduction Worldwide, tuberculosis is the most common serious opportunistic infection among people with HIV infection. -
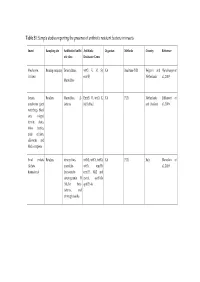
Table S1: Sample Studies Reporting the Presence of Antibiotic Resistant Bacteria in Insects
Table S1: Sample studies reporting the presence of antibiotic resistant bacteria in insects Insect Sampling site Antibiotics/Antibi Antibiotic Organism Methods Country Reference otic class Resistance Genes Mealworm, Rearing company Tetracyclines, tet(O, K, M, S), NA Real-time PCR Belgium and (Vandeweyer et Crickets erm(B) Netherlands al., 2019) Macrolides locusts, Retailers Macrolides; β- Erm(B, C), tet(O, K, NA PCR Netherlands (Milanović et mealworm giant lactams M, S), blaZ and Thailand al., 2016) waterbugs, black ants, winged termite alates, rhino beetles, mole crickets, silkworm, and black scorpions Small crickets Retailers tetracyclines, tet(M), tet(O), tet(K), NA PCR Italy (Roncolini et (Acheta macrolide- tet(S), erm(B), al., 2019) domesticus) lincosamide- erm(C), blaZ and streptogramin B mecA, aac(6')-Ie (MLSB), beta- aph(2")-Ia lactams, and aminoglycosides Cockroach Hospital Penicillins, blaZ, aacA-D, tetK, Staphylococcus Culture, PCR Iran (Abdolmaleki environment Cephems, msrA, dfrA, msrA, aureus et al., 2019) Aminoglycosides, vatB, tetM, grlA, macrolides. Folate rpoB, vatA, linA, inhibitors, ermA, cat1 Tetracyclines, fluoroquinolones, Lincosamides, Phenicols, Ansamycins Cockroaches Household ampicillin, NA Enterobacter spp., Culture Iran (Vazirianzadeh kitches cephalothin, Klebsiella et al., 2014) ceftriaxone, spp., Citrobacter ciproflexoxacin, spp., E. coli, chloramphenicol, Salmonella spp., gentamicin, Proteus spp., tetracycline, coagulase negative trimethoprim- staph- ylococci, S. sulfamethoxazole, marcescens, ceftazidime, -

Spironolactone
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Spironolactone This information sheet should be read in conjunction with any information provided by the manufacturer. This information sheet explains what spironolactone is, how it is given and some of the possible side effects. Each person reacts differently to medicines, so your child will not necessarily suffer every side effect mentioned. If you have any questions or concerns, please ask your doctor, nurse or pharmacist or telephone one of the contact numbers on this information sheet. What is spironolactone? The liquid preparation comes in 5mg/ml, 10mg/5ml, 25mg/5ml, 50mg/5ml and 100mg/5ml Spironolactone belongs to a group of medicines strengths. You should use the oral syringe called diuretics. It is commonly used alongside provided to draw up the correct amount of another medicine called furosemide to reduce liquid and squirt it in the inside of your child’s fluid overload, so reducing the amount of work cheek. Please note that some of these strengths the heart has to do to pump blood around the may not be available locally so you should body. It works by making the kidneys produce always tell the pharmacist the strength of your more urine (wee), so your child may have to child’s usual medicine. go to the toilet more often. It also prevents potassium being lost in the urine. It is commonly Your doctor, nurse or pharmacist will have prescribed for children with heart or kidney explained to you how to obtain the correct problems causing excess fluid in the body. -

Therapy of Rhodococcus Equi Infections in Foals
IN-DEPTH: RHODOCOCCUS EQUI Therapy of Rhodococcus equi Infections in Foals Steeve Gigue`re, DVM, PhD, Diplomate ACVIM The combination of a macrolide (erythromycin, azithromycin, or clarithromycin) with rifampin re- mains the mainstay of therapy. Although the vast majority of Rhodococcus equi isolates are still susceptible to these antimicrobials, the frequency of macrolide-rifampin–resistant isolates seems to be increasing. Author’s address: College of Veterinary Medicine, University of Georgia, Athens, Georgia 30602; e-mail: [email protected]. © 2010 AAEP. 1. Introduction lates are inhibited (MIC90) is 0.12, 0.25, and A wide variety of antimicrobial agents are active 1.0 g/ml for clarithromycin, erythromycin, and 5 against Rhodococcus equi in vitro. However, be- azithromycin, respectively. The combination of a cause R. equi is a facultative intracellular pathogen macrolide and rifampin is synergistic both in vitro surviving and replicating in macrophages, many of and in vivo, and the use of the two classes of drugs in these drugs are ineffective in vivo. For example, in combination reduces the likelihood of R. equi resis- one study, all 17 foals with R. equi pneumonia tance to either drug.4,6 Rifampin and macrolides treated with the combination of penicillin and gen- are lipid soluble, allowing them to penetrate cell tamicin died despite the fact that all isolates were membranes and caseous material. sensitive to gentamicin.1 The combination of ri- The recommended doses are listed in Table fampin and erythromycin became the treatment of 1. Several formulations of erythromycin are com- choice in the 1980s and has dramatically reduced mercially available. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2019 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

Alphabetical Listing of ATC Drugs & Codes
Alphabetical Listing of ATC drugs & codes. Introduction This file is an alphabetical listing of ATC codes as supplied to us in November 1999. It is supplied free as a service to those who care about good medicine use by mSupply support. To get an overview of the ATC system, use the “ATC categories.pdf” document also alvailable from www.msupply.org.nz Thanks to the WHO collaborating centre for Drug Statistics & Methodology, Norway, for supplying the raw data. I have intentionally supplied these files as PDFs so that they are not quite so easily manipulated and redistributed. I am told there is no copyright on the files, but it still seems polite to ask before using other people’s work, so please contact <[email protected]> for permission before asking us for text files. mSupply support also distributes mSupply software for inventory control, which has an inbuilt system for reporting on medicine usage using the ATC system You can download a full working version from www.msupply.org.nz Craig Drown, mSupply Support <[email protected]> April 2000 A (2-benzhydryloxyethyl)diethyl-methylammonium iodide A03AB16 0.3 g O 2-(4-chlorphenoxy)-ethanol D01AE06 4-dimethylaminophenol V03AB27 Abciximab B01AC13 25 mg P Absorbable gelatin sponge B02BC01 Acadesine C01EB13 Acamprosate V03AA03 2 g O Acarbose A10BF01 0.3 g O Acebutolol C07AB04 0.4 g O,P Acebutolol and thiazides C07BB04 Aceclidine S01EB08 Aceclidine, combinations S01EB58 Aceclofenac M01AB16 0.2 g O Acefylline piperazine R03DA09 Acemetacin M01AB11 Acenocoumarol B01AA07 5 mg O Acepromazine N05AA04 -

Summary of Product Characteristics
Annex I SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Syndi-35 2 mg+ 0,035 mg, coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 2 milligrams cyproterone acetate (Cyproteroni acetas) and 35 micrograms of ethinylestradiol (Ethinylestradiolum). For full list of excipients, see 6.1. 3. PHARMACEUTICAL FORM Coated tablets. Round, biconvex, yellow sugar-coated tablets with a 5.7 mm nominal diameter. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Syndi-35,coated tablets is indicated for use only in women for the treatment of: (a) severe acne, refractory to prolonged oral antibiotic therapy; (b) moderately severe hirsutism. Although Syndi-35, coated tablets also acts as an oral contraceptive, it is not recommended solely for contraception, but should be reserved for women requiring treatment for the androgendependent skin conditions described. 4.2 Posology and method of administration First treatment course One tablet daily for 21 days, starting on the first day of the menstrual cycle (the first day of menstruation counting as Day 1). Subsequent courses Each subsequent course is started 7 tablet-free days after the preceding course. Complete remission of acne is to be expected in nearly all cases, often within a few months, but in particularly severe cases treatment for longer periods may be necessary before the full benefit is seen. It is recommended that treatment be withdrawn when the acne or hirsutism has completely resolved. Repeat courses of Syndi-35 coated tablets may be given if the condition recurs. When the contraceptive action of Syndi-35 coated tablets is also to be employed, it is essential that the above instructions be rigidly adhered to. -

CYPRONE 50 Cyproterone Acetate Tablets
AUSTRALIAN PRODUCT INFORMATION CYPRONE 50 Cyproterone acetate tablets 1 NAME OF THE MEDICINE Cyproterone acetate 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each Cyprone 50 tablet contains 50 mg of cyproterone acetate as the active ingredient. Excipients with known effect: Contains sugars (as lactose). For the full list of excipients, see Section 6.1 LIST OF EXCIPIENTS. 3 PHARMACEUTICAL FORM Cyprone 50 : White circular flat bevelled tablets, with breakline on one side and plain on the other. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Women Moderately severe to severe signs of androgenization • moderately severe/severe forms of hirsutism • moderately severe/severe androgen‐dependent loss of scalp hair (moderately severe/severe androgenetic alopecia) • moderately severe/severe forms of acne and/or seborrhoea associated with other features of androgenization Cyproterone acetate inhibits the influence of male sex hormones which are also produced by the female. It is thus possible to treat diseases in women caused either by increased production of androgens or a particular sensitivity to these hormones. Hirsutism and alopecia may be expected to recur over a period of time after cessation of treatment. If Cyprone 50 is taken during pregnancy, the properties of the preparation may lead to signs of feminisation in the male foetus. Therefore, in women of childbearing potential, pregnancy must be excluded at the commencement of treatment and ethinylestradiol taken as well to ensure contraception. This also promotes regular menstruation. Men Reduction of drive in sexual deviations Cyprone 50 reduces the force of the sexual urge in men with sexual deviations. Whilst under treatment, the man can control himself better in a predisposing stimulatory situation, but there is no influence on any deviating direction of sexual drive. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0