Clinical Uses and Control of Rifampicin and Clindamycin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RIFAMPICIN Productinformation Sigma Prod
RIFAMPICIN ProductInformation Sigma Prod. No. R3501 CH3 CH3 CAS NUMBER: 13292-46-1 HO SYNONYMS: Tubocin; Sinerdol; Rimactan; L-5103; Dione-21 Acetate; Archidyn; Arficin; 3-(4- CH3 O O OH O Methylpiperazinyliminomethyl)-rifamycin SV; NSC 113926; C OH OH CH 1 2 3 H C Rifampin ; Rifaldazine; Rifamycin AMP H3C 3 O NH H3C PHYSICAL PROPERTIES: CH3 N CH N Appearance: Orange-brown to red-brown powder.3 O OH N Molecular formula: C43H58N4O12 O Molecular weight: 823.0 O CH3 CH3 EmM (max absorbance, phosphate buffer, pH 7.38): 33.20 (237 nm); 32.10 (255 nm); 27.00 (334 nm); 15.40 (475 nm)2,4 pKa (in water):1.7 (4-hydroxyl group), 7.9 (4-piperazine nitrogen); in methylcellosolve-water (4:1): 3.6 (4- hydroxyl group), 6.7 (3-piperazine nitrogen)4 pI (in water): 4.84 25° 4 Optical rotation: [α]D =+10.6° (c=0.5% in CDCl3) Melting point: 183-188°C (dec.)2,4 METHOD OF PREPARATION: Methods of preparation have been reported.4,5 The NMR, UV, IR, Mass spectra, Thin-Layer chromatography and HPLC methods of detection have been reported.4,5,6 A colorimetric test for identification was reported.4 STABILITY / STORAGE: Rifampicin (Rif) should be stable for at least two years when stored desiccated at -20°C and protected from light.3 Rif is stable as a solid at temperatures up to 70EC.4 SOLUBILITY / SOLUTION STABILITY: Rif is soluble in dimethylsulfoxide (~100mg/mL), dimethylformamide, methanol (16 mg/ml, 25EC), chloroform (349 mg/ml, 25°C), ethyl acetate (108 mg/ml, 25°C), and acetone (14 mg/ml, 25°C).4,6,7,8,9 Rif is slightly soluble in water at 25°C: 2.5 mg/ml, pH 7.3; 1.3 mg/ml, pH 4.3; and in 95% ethanol (∼10 mg/mL).4 Rif is soluble at 37°C: in 0.1 N HCl, 200 mg/ml and in phosphate buffer pH 7.4, 9.9 mg/ml.4 R3501 Page 1 of 4 03/28/97 - ARO RIFAMPICIN Sigma Prod. -

PROCUR Why Procur Has Been Prescribed for You
Consumer Medicine Information Ask your doctor if you have any questions about PROCUR why Procur has been prescribed for you. Cyproterone acetate 50 mg and 100 mg tablets This medicine is available only with a doctor's prescription. What is in this leaflet Before you take Procur Please read this leaflet carefully before you start taking Procur When you must not take it This leaflet answers some common questions about Procur. It does not contain all the available Do not take Procur if you have an allergy to: information. It does not take the place of talking • any medicine containing cyproterone acetate to your doctor or pharmacist. • any of the ingredients listed at the end of this leaflet All medicines have risks and benefits. Your doctor has weighed the risks of you taking Procur against Some of the symptoms of an allergic reaction may the benefits they expect it will have for you. include: • difficulty in breathing or wheezing If you have any concerns about taking this • shortness of breath medicine, ask your doctor or pharmacist. • swelling of the face, tongue, lips, or other parts of the body Keep this leaflet with the medicine. You may • hives on the skin, rash, or itching need to read it again. Do not take Procur if: What Procur is used for • you are allergic to cyproterone acetate or any other ingredient listed at the end of this leaflet Procur tablets contain the active ingredient • you are pregnant cyproterone acetate. Cyproterone acetate is an • you are breastfeeding antiandrogen. It works by blocking the actions of • you suffer from liver diseases (including sex hormones (androgens) that are produced previous or existing liver tumours, Dubin- mainly in men but also, to a lesser extent in Johnson syndrome or Rotor syndrome) women. -

Oxytetracycline for Recurrent Blepharitis and Br J Ophthalmol: First Published As 10.1136/Bjo.79.1.42 on 1 January 1995
42 British rournal of Ophthalmology 1995; 79: 42-45 Placebo controlled trial of fusidic acid gel and oxytetracycline for recurrent blepharitis and Br J Ophthalmol: first published as 10.1136/bjo.79.1.42 on 1 January 1995. Downloaded from rosacea D V Seal, P Wright, L Ficker, K Hagan, M Troski, P Menday Abstract topical and systemic anti-staphylococcal A prospective, randomised, double blind, antibiotics in addition to anti-inflammatory partial crossover, placebo controlled trial therapy. has been conducted to compare the Fusidic acid has been in clinical use since performance of topical fusidic acid gel 1962 and is particularly effective against (Fucithalmic) and oral oxytetracycline as staphylococci. It has a steroid structure but no treatment for symptomatic chronic glucocorticoid activity. A new gel preparation blepharitis. Treatment success was containing 1% microcrystalline fusidic acid judged both by a reduction in symptoms (Fucithalmic) has recently been shown to be and clinical examination before and after effective for treating acute bacterial conjunc- therapy. Seventy five per cent of patients tivitis and for reducing the staphylococcal lid with blepharitis and associated rosacea flora before surgery.5-8 were symptomatically improved by Oxytetracycline has been selected for the fusidic acid gel and 500/0 by oxytetra- treatment of patients with chronic blepharitis cycline, but fewer (35%/o) appeared to as it has both anti-inflammatory and anti- benefit from the combination. Patients staphylococcal properties.9 10 It has been with chronic blepharitis of other aetiolo- demonstrated to be effective in a controlled gies did not respond to fusidic acid gel but trial in ocular rosacea for comeal infiltrates and 25% did benefit from oxytetracycline and conjunctival hyperaemiaII and in non-ocular 300/0 from the combination. -

Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
pharmaceutics Review Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Luíse L. Chaves 1,2,*, Yuri Patriota 2, José L. Soares-Sobrinho 2 , Alexandre C. C. Vieira 1,3, Sofia A. Costa Lima 1,4 and Salette Reis 1,* 1 Laboratório Associado para a Química Verde, Rede de Química e Tecnologia, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, 4050-313 Porto, Portugal; [email protected] (A.C.C.V.); slima@ff.up.pt (S.A.C.L.) 2 Núcleo de Controle de Qualidade de Medicamentos e Correlatos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil; [email protected] (Y.P.); [email protected] (J.L.S.-S.) 3 Laboratório de Tecnologia dos Medicamentos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil 4 Cooperativa de Ensino Superior Politécnico e Universitário, Instituto Universitário de Ciências da Saúde, 4585-116 Gandra, Portugal * Correspondence: [email protected] (L.L.C.); shreis@ff.up.pt (S.R.) Received: 30 October 2020; Accepted: 4 December 2020; Published: 11 December 2020 Abstract: Leprosy disease remains an important public health issue as it is still endemic in several countries. Mycobacterium leprae, the causative agent of leprosy, presents tropism for cells of the reticuloendothelial and peripheral nervous system. Current multidrug therapy consists of clofazimine, dapsone and rifampicin. Despite significant improvements in leprosy treatment, in most programs, successful completion of the therapy is still sub-optimal. Drug resistance has emerged in some countries. This review discusses the status of leprosy disease worldwide, providing information regarding infectious agents, clinical manifestations, diagnosis, actual treatment and future perspectives and strategies on targets for an efficient targeted delivery therapy. -

Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis
Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Centers for Disease Control and Prevention Office of Infectious Diseases National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination June 2013 This document is accessible online at http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Suggested citation: CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online]. 2013. Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Table of Contents Introduction 1 Methodology for Preparation of these Guidelines 2 The Role of Rifamycins in Tuberculosis Treatment 4 Managing Drug Interactions with Antivirals and Rifampin 5 Managing Drug Interactions with Antivirals and Rifabutin 9 Treatment of Latent TB Infection with Rifampin or Rifapentine 10 Treating Pregnant Women with Tuberculosis and HIV Co-infection 10 Treating Children with HIV-associated Tuberculosis 12 Co-treatment of Multidrug-resistant Tuberculosis and HIV 14 Limitations of these Guidelines 14 HIV-TB Drug Interaction Guideline Development Group 15 References 17 Table 1a. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in adults 21 Table 1b. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in children 22 Table 2a. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in adults 23 Table 2b. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in children 25 Table 3. Recommendations for co-administering antiretroviral drugs with RIFABUTIN in adults 26 ii Introduction Worldwide, tuberculosis is the most common serious opportunistic infection among people with HIV infection. -

(OTC) Antibiotics in the European Union and Norway, 2012
Perspective Analysis of licensed over-the-counter (OTC) antibiotics in the European Union and Norway, 2012 L Both 1 , R Botgros 2 , M Cavaleri 2 1. Public Health England (PHE), London, United Kingdom 2. Anti-infectives and Vaccines Office, European Medicines Agency (EMA), London, United Kingdom Correspondence: Marco Cavaleri ([email protected]) Citation style for this article: Both L, Botgros R, Cavaleri M. Analysis of licensed over-the-counter (OTC) antibiotics in the European Union and Norway, 2012. Euro Surveill. 2015;20(34):pii=30002. DOI: http://dx.doi.org/10.2807/1560-7917.ES.2015.20.34.30002 Article submitted on 16 September 2014 / accepted on 09 February 2015 / published on 27 August 2015 Antimicrobial resistance is recognised as a growing throughout the EU; however, there are still consider- problem that seriously threatens public health and able differences in Europe due to the different health- requires prompt action. Concerns have therefore been care structures and policies (including the extent of raised about the potential harmful effects of making pharmacist supervision for OTC medicines), reimburse- antibiotics available without prescription. Because of ment policies, and cultural differences of each Member the very serious concerns regarding further spread of State. Therefore, the availability of OTC medicines var- resistance, the over-the-counter (OTC) availability of ies in the EU and products sold as POM in certain coun- antibiotics was analysed here. Topical and systemic tries can be obtained as OTC medicines in others. OTC antibiotics and their indications were determined across 26 European Union (EU) countries and Norway As risk minimisation is an important criterion for some by means of a European survey. -

Withdrawal Times of Oxytetracycline and Tylosin in Eggs of Laying Hens After Oral Administration
1017 Journal of Food Protection, Vol. 77, No. 6, 2014, Pages 1017–1021 doi:10.4315/0362-028X.JFP-13-440 Copyright G, International Association for Food Protection Research Note Withdrawal Times of Oxytetracycline and Tylosin in Eggs of Laying Hens after Oral Administration RUBE´ N MUN˜ OZ,1 JAVIERA CORNEJO,2 ALDO MADDALENO,1 CAROLINA ARAYA-JORDA´ N,1 DANIELA IRAGU¨ EN,1 NICOLA´ S PIZARRO,1 AND BETTY SAN MARTI´N1* 1 2 Laboratory of Veterinary Pharmacology and Department of Animal Preventive Medicine, Faculty of Veterinary and Animal Sciences, Downloaded from http://meridian.allenpress.com/jfp/article-pdf/77/6/1017/1687041/0362-028x_jfp-13-440.pdf by guest on 30 September 2021 Universidad de Chile, Avenida Santa Rosa 11735, La Pintana, Santiago, Chile MS 13-440: Received 3 October 2013/Accepted 3 January 2014 ABSTRACT Antimicrobials administered to laying hens may be distributed into egg white or yolk, indicating the importance of evaluating withdrawal times (WDTs) of the pharmaceutical formulations. In the present study, oxytetracycline and tylosin’s WDTs were estimated. The concentration and depletion of these molecules in eggs were linked to their pharmacokinetic and physicochemical properties. Twenty-seven Leghorn hens were used: 12 treated with oxytetracycline, 12 treated with tylosin, and 3 remained as an untreated control group. After completion of therapies, eggs were collected daily and drug concentrations in egg white and yolk were assessed. The yolk was used as the target tissue to evaluate the WDT; the results were 9 and 3 days for oxytetracycline and tylosin, respectively. In particular, oxytetracycline has a good oral bioavailability, a moderate apparent volume of distribution, a molecular weight of 460 g/mol, and is lightly liposoluble. -

Blepharitis Last Revised in October 2015
Menu Sign in (https://accounts.nice.org.uk/signin) Search... Blepharitis Last revised in October 2015 Changes Back to top Last revised in October 2015 October 2015 — reviewed. A literature search was conducted in October 2015 to identify evidence based guidelines, UK policy, systematic reviews, and key randomized controlled trials published since the last revision of the topic. No changes to clinical recommendations have been made. Previous changes Back to top September 2012 — reviewed. A literature search was conducted in September 2012 to identify evidencebased guidelines, UK policy, systematic reviews, and key RCTs published since the last revision of the topic. No changes to clinical recommendations have been made. April 2011 — minor update. Change to recommendation regarding need for additional contraception during or after a course of tetracycline additional contraception is no longer required when using antibiotics that are not enzyme inducers with combined hormonal methods for durations of 3 weeks or less [FSRH, 2011 (/blepharitis#!references/315093)]. Issued in June 2011. March 2011 — topic structure revised to ensure consistency across CKS topics — no changes to clinical recommendations have been made. September 2010 — minor update. Hydromoor® (hypromellose 0.3% preservativefree single dose eye drops) have been included. Issued in September 2010. March 2010 — minor update. LubriTears® eye ointment has been discontinued. Prescription removed. Issued in March 2010. December 2007 to May 2008 — converted from CKS guidance to CKS topic structure. The evidence base has been reviewed in detail, and recommendations are more clearly justified and transparently linked to the supporting evidence. The clinical scenario structure has been reviewed. -

(ESVAC) Web-Based Sales and Animal Population
16 July 2019 EMA/210691/2015-Rev.2 Veterinary Medicines Division European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) Sales Data and Animal Population Data Collection Protocol (version 3) Superseded by a new version Superseded Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. Reproduction is authorised provided the source is acknowledged. Table of content 1. Introduction ....................................................................................................................... 3 1.1. Terms of reference ........................................................................................................... 3 1.2. Approach ........................................................................................................................ 3 1.3. Target groups of the protocol and templates ......................................................................... 4 1.4. Organization of the ESVAC project ...................................................................................... 4 1.5. Web based delivery of data ................................................................................................ 5 2. ESVAC sales data ............................................................................................................... 5 2.1. -
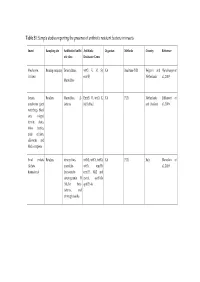
Table S1: Sample Studies Reporting the Presence of Antibiotic Resistant Bacteria in Insects
Table S1: Sample studies reporting the presence of antibiotic resistant bacteria in insects Insect Sampling site Antibiotics/Antibi Antibiotic Organism Methods Country Reference otic class Resistance Genes Mealworm, Rearing company Tetracyclines, tet(O, K, M, S), NA Real-time PCR Belgium and (Vandeweyer et Crickets erm(B) Netherlands al., 2019) Macrolides locusts, Retailers Macrolides; β- Erm(B, C), tet(O, K, NA PCR Netherlands (Milanović et mealworm giant lactams M, S), blaZ and Thailand al., 2016) waterbugs, black ants, winged termite alates, rhino beetles, mole crickets, silkworm, and black scorpions Small crickets Retailers tetracyclines, tet(M), tet(O), tet(K), NA PCR Italy (Roncolini et (Acheta macrolide- tet(S), erm(B), al., 2019) domesticus) lincosamide- erm(C), blaZ and streptogramin B mecA, aac(6')-Ie (MLSB), beta- aph(2")-Ia lactams, and aminoglycosides Cockroach Hospital Penicillins, blaZ, aacA-D, tetK, Staphylococcus Culture, PCR Iran (Abdolmaleki environment Cephems, msrA, dfrA, msrA, aureus et al., 2019) Aminoglycosides, vatB, tetM, grlA, macrolides. Folate rpoB, vatA, linA, inhibitors, ermA, cat1 Tetracyclines, fluoroquinolones, Lincosamides, Phenicols, Ansamycins Cockroaches Household ampicillin, NA Enterobacter spp., Culture Iran (Vazirianzadeh kitches cephalothin, Klebsiella et al., 2014) ceftriaxone, spp., Citrobacter ciproflexoxacin, spp., E. coli, chloramphenicol, Salmonella spp., gentamicin, Proteus spp., tetracycline, coagulase negative trimethoprim- staph- ylococci, S. sulfamethoxazole, marcescens, ceftazidime, -

Third ESVAC Report
Sales of veterinary antimicrobial agents in 25 EU/EEA countries in 2011 Third ESVAC report An agency of the European Union The mission of the European Medicines Agency is to foster scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health. Legal role Guiding principles The European Medicines Agency is the European Union • We are strongly committed to public and animal (EU) body responsible for coordinating the existing health. scientific resources put at its disposal by Member States • We make independent recommendations based on for the evaluation, supervision and pharmacovigilance scientific evidence, using state-of-the-art knowledge of medicinal products. and expertise in our field. • We support research and innovation to stimulate the The Agency provides the Member States and the development of better medicines. institutions of the EU the best-possible scientific advice on any question relating to the evaluation of the quality, • We value the contribution of our partners and stake- safety and efficacy of medicinal products for human or holders to our work. veterinary use referred to it in accordance with the • We assure continual improvement of our processes provisions of EU legislation relating to medicinal prod- and procedures, in accordance with recognised quality ucts. standards. • We adhere to high standards of professional and Principal activities personal integrity. Working with the Member States and the European • We communicate in an open, transparent manner Commission as partners in a European medicines with all of our partners, stakeholders and colleagues. network, the European Medicines Agency: • We promote the well-being, motivation and ongoing professional development of every member of the • provides independent, science-based recommenda- Agency. -

Spironolactone
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Spironolactone This information sheet should be read in conjunction with any information provided by the manufacturer. This information sheet explains what spironolactone is, how it is given and some of the possible side effects. Each person reacts differently to medicines, so your child will not necessarily suffer every side effect mentioned. If you have any questions or concerns, please ask your doctor, nurse or pharmacist or telephone one of the contact numbers on this information sheet. What is spironolactone? The liquid preparation comes in 5mg/ml, 10mg/5ml, 25mg/5ml, 50mg/5ml and 100mg/5ml Spironolactone belongs to a group of medicines strengths. You should use the oral syringe called diuretics. It is commonly used alongside provided to draw up the correct amount of another medicine called furosemide to reduce liquid and squirt it in the inside of your child’s fluid overload, so reducing the amount of work cheek. Please note that some of these strengths the heart has to do to pump blood around the may not be available locally so you should body. It works by making the kidneys produce always tell the pharmacist the strength of your more urine (wee), so your child may have to child’s usual medicine. go to the toilet more often. It also prevents potassium being lost in the urine. It is commonly Your doctor, nurse or pharmacist will have prescribed for children with heart or kidney explained to you how to obtain the correct problems causing excess fluid in the body.