CYPRONE 50 Cyproterone Acetate Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

35 Cyproterone Acetate and Ethinyl Estradiol Tablets 2 Mg/0
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrCYESTRA®-35 cyproterone acetate and ethinyl estradiol tablets 2 mg/0.035 mg THERAPEUTIC CLASSIFICATION Acne Therapy Paladin Labs Inc. Date of Preparation: 100 Alexis Nihon Blvd, Suite 600 January 17, 2019 St-Laurent, Quebec H4M 2P2 Version: 6.0 Control # 223341 _____________________________________________________________________________________________ CYESTRA-35 Product Monograph Page 1 of 48 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION ....................................................................... 3 SUMMARY PRODUCT INFORMATION ............................................................................................. 3 INDICATION AND CLINICAL USE ..................................................................................................... 3 CONTRAINDICATIONS ........................................................................................................................ 3 WARNINGS AND PRECAUTIONS ....................................................................................................... 4 ADVERSE REACTIONS ....................................................................................................................... 13 DRUG INTERACTIONS ....................................................................................................................... 16 DOSAGE AND ADMINISTRATION ................................................................................................ 20 OVERDOSAGE .................................................................................................................................... -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

RIFAMPICIN Productinformation Sigma Prod
RIFAMPICIN ProductInformation Sigma Prod. No. R3501 CH3 CH3 CAS NUMBER: 13292-46-1 HO SYNONYMS: Tubocin; Sinerdol; Rimactan; L-5103; Dione-21 Acetate; Archidyn; Arficin; 3-(4- CH3 O O OH O Methylpiperazinyliminomethyl)-rifamycin SV; NSC 113926; C OH OH CH 1 2 3 H C Rifampin ; Rifaldazine; Rifamycin AMP H3C 3 O NH H3C PHYSICAL PROPERTIES: CH3 N CH N Appearance: Orange-brown to red-brown powder.3 O OH N Molecular formula: C43H58N4O12 O Molecular weight: 823.0 O CH3 CH3 EmM (max absorbance, phosphate buffer, pH 7.38): 33.20 (237 nm); 32.10 (255 nm); 27.00 (334 nm); 15.40 (475 nm)2,4 pKa (in water):1.7 (4-hydroxyl group), 7.9 (4-piperazine nitrogen); in methylcellosolve-water (4:1): 3.6 (4- hydroxyl group), 6.7 (3-piperazine nitrogen)4 pI (in water): 4.84 25° 4 Optical rotation: [α]D =+10.6° (c=0.5% in CDCl3) Melting point: 183-188°C (dec.)2,4 METHOD OF PREPARATION: Methods of preparation have been reported.4,5 The NMR, UV, IR, Mass spectra, Thin-Layer chromatography and HPLC methods of detection have been reported.4,5,6 A colorimetric test for identification was reported.4 STABILITY / STORAGE: Rifampicin (Rif) should be stable for at least two years when stored desiccated at -20°C and protected from light.3 Rif is stable as a solid at temperatures up to 70EC.4 SOLUBILITY / SOLUTION STABILITY: Rif is soluble in dimethylsulfoxide (~100mg/mL), dimethylformamide, methanol (16 mg/ml, 25EC), chloroform (349 mg/ml, 25°C), ethyl acetate (108 mg/ml, 25°C), and acetone (14 mg/ml, 25°C).4,6,7,8,9 Rif is slightly soluble in water at 25°C: 2.5 mg/ml, pH 7.3; 1.3 mg/ml, pH 4.3; and in 95% ethanol (∼10 mg/mL).4 Rif is soluble at 37°C: in 0.1 N HCl, 200 mg/ml and in phosphate buffer pH 7.4, 9.9 mg/ml.4 R3501 Page 1 of 4 03/28/97 - ARO RIFAMPICIN Sigma Prod. -

PROCUR Why Procur Has Been Prescribed for You
Consumer Medicine Information Ask your doctor if you have any questions about PROCUR why Procur has been prescribed for you. Cyproterone acetate 50 mg and 100 mg tablets This medicine is available only with a doctor's prescription. What is in this leaflet Before you take Procur Please read this leaflet carefully before you start taking Procur When you must not take it This leaflet answers some common questions about Procur. It does not contain all the available Do not take Procur if you have an allergy to: information. It does not take the place of talking • any medicine containing cyproterone acetate to your doctor or pharmacist. • any of the ingredients listed at the end of this leaflet All medicines have risks and benefits. Your doctor has weighed the risks of you taking Procur against Some of the symptoms of an allergic reaction may the benefits they expect it will have for you. include: • difficulty in breathing or wheezing If you have any concerns about taking this • shortness of breath medicine, ask your doctor or pharmacist. • swelling of the face, tongue, lips, or other parts of the body Keep this leaflet with the medicine. You may • hives on the skin, rash, or itching need to read it again. Do not take Procur if: What Procur is used for • you are allergic to cyproterone acetate or any other ingredient listed at the end of this leaflet Procur tablets contain the active ingredient • you are pregnant cyproterone acetate. Cyproterone acetate is an • you are breastfeeding antiandrogen. It works by blocking the actions of • you suffer from liver diseases (including sex hormones (androgens) that are produced previous or existing liver tumours, Dubin- mainly in men but also, to a lesser extent in Johnson syndrome or Rotor syndrome) women. -

Ritonavir Mylan, INN-Ritonavir
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Ritonavir Mylan 100 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 100 mg of ritonavir. Excipient with known effect Each film-coated tablet contains 87.75 mg of sodium. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Film-coated tablet (tablet). Yellow, capsule shaped, biconvex, beveled edge film-coated tablet, approximately 19.1 mm x 10.2 mm, debossed with ‘M163’ on one side and blank on the other side. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Ritonavir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infected patients (adults and children of 2 years of age and older). 4.2 Posology and method of administration Ritonavir Mylan should be administered by physicians who are experienced in the treatment of HIV infection. Posology Ritonavir dosed as a pharmacokinetic enhancer When ritonavir is used as a pharmacokinetic enhancer with other protease inhibitors the Summary of Product Characteristics for the particular protease inhibitor must be consulted. The following HIV-1 protease inhibitors have been approved for use with ritonavir as a pharmacokinetic enhancer at the noted doses. Adults Amprenavir 600 mg twice daily with ritonavir 100 mg twice daily. Atazanavir 300 mg once daily with ritonavir 100 mg once daily. Fosamprenavir 700 mg twice daily with ritonavir 100 mg twice daily. Lopinavir co-formulated with ritonavir (lopinavir/ritonavir) 400 mg/100 mg or 800 mg/200 mg. Saquinavir 1,000 mg twice daily with ritonavir 100 mg twice daily in ART experienced patients. -

Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
pharmaceutics Review Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Luíse L. Chaves 1,2,*, Yuri Patriota 2, José L. Soares-Sobrinho 2 , Alexandre C. C. Vieira 1,3, Sofia A. Costa Lima 1,4 and Salette Reis 1,* 1 Laboratório Associado para a Química Verde, Rede de Química e Tecnologia, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, 4050-313 Porto, Portugal; [email protected] (A.C.C.V.); slima@ff.up.pt (S.A.C.L.) 2 Núcleo de Controle de Qualidade de Medicamentos e Correlatos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil; [email protected] (Y.P.); [email protected] (J.L.S.-S.) 3 Laboratório de Tecnologia dos Medicamentos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil 4 Cooperativa de Ensino Superior Politécnico e Universitário, Instituto Universitário de Ciências da Saúde, 4585-116 Gandra, Portugal * Correspondence: [email protected] (L.L.C.); shreis@ff.up.pt (S.R.) Received: 30 October 2020; Accepted: 4 December 2020; Published: 11 December 2020 Abstract: Leprosy disease remains an important public health issue as it is still endemic in several countries. Mycobacterium leprae, the causative agent of leprosy, presents tropism for cells of the reticuloendothelial and peripheral nervous system. Current multidrug therapy consists of clofazimine, dapsone and rifampicin. Despite significant improvements in leprosy treatment, in most programs, successful completion of the therapy is still sub-optimal. Drug resistance has emerged in some countries. This review discusses the status of leprosy disease worldwide, providing information regarding infectious agents, clinical manifestations, diagnosis, actual treatment and future perspectives and strategies on targets for an efficient targeted delivery therapy. -

Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis
Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Centers for Disease Control and Prevention Office of Infectious Diseases National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination June 2013 This document is accessible online at http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Suggested citation: CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online]. 2013. Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Table of Contents Introduction 1 Methodology for Preparation of these Guidelines 2 The Role of Rifamycins in Tuberculosis Treatment 4 Managing Drug Interactions with Antivirals and Rifampin 5 Managing Drug Interactions with Antivirals and Rifabutin 9 Treatment of Latent TB Infection with Rifampin or Rifapentine 10 Treating Pregnant Women with Tuberculosis and HIV Co-infection 10 Treating Children with HIV-associated Tuberculosis 12 Co-treatment of Multidrug-resistant Tuberculosis and HIV 14 Limitations of these Guidelines 14 HIV-TB Drug Interaction Guideline Development Group 15 References 17 Table 1a. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in adults 21 Table 1b. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in children 22 Table 2a. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in adults 23 Table 2b. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in children 25 Table 3. Recommendations for co-administering antiretroviral drugs with RIFABUTIN in adults 26 ii Introduction Worldwide, tuberculosis is the most common serious opportunistic infection among people with HIV infection. -
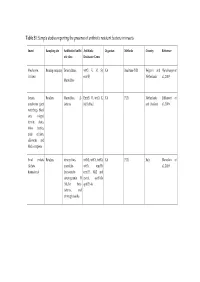
Table S1: Sample Studies Reporting the Presence of Antibiotic Resistant Bacteria in Insects
Table S1: Sample studies reporting the presence of antibiotic resistant bacteria in insects Insect Sampling site Antibiotics/Antibi Antibiotic Organism Methods Country Reference otic class Resistance Genes Mealworm, Rearing company Tetracyclines, tet(O, K, M, S), NA Real-time PCR Belgium and (Vandeweyer et Crickets erm(B) Netherlands al., 2019) Macrolides locusts, Retailers Macrolides; β- Erm(B, C), tet(O, K, NA PCR Netherlands (Milanović et mealworm giant lactams M, S), blaZ and Thailand al., 2016) waterbugs, black ants, winged termite alates, rhino beetles, mole crickets, silkworm, and black scorpions Small crickets Retailers tetracyclines, tet(M), tet(O), tet(K), NA PCR Italy (Roncolini et (Acheta macrolide- tet(S), erm(B), al., 2019) domesticus) lincosamide- erm(C), blaZ and streptogramin B mecA, aac(6')-Ie (MLSB), beta- aph(2")-Ia lactams, and aminoglycosides Cockroach Hospital Penicillins, blaZ, aacA-D, tetK, Staphylococcus Culture, PCR Iran (Abdolmaleki environment Cephems, msrA, dfrA, msrA, aureus et al., 2019) Aminoglycosides, vatB, tetM, grlA, macrolides. Folate rpoB, vatA, linA, inhibitors, ermA, cat1 Tetracyclines, fluoroquinolones, Lincosamides, Phenicols, Ansamycins Cockroaches Household ampicillin, NA Enterobacter spp., Culture Iran (Vazirianzadeh kitches cephalothin, Klebsiella et al., 2014) ceftriaxone, spp., Citrobacter ciproflexoxacin, spp., E. coli, chloramphenicol, Salmonella spp., gentamicin, Proteus spp., tetracycline, coagulase negative trimethoprim- staph- ylococci, S. sulfamethoxazole, marcescens, ceftazidime, -

Spironolactone
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Spironolactone This information sheet should be read in conjunction with any information provided by the manufacturer. This information sheet explains what spironolactone is, how it is given and some of the possible side effects. Each person reacts differently to medicines, so your child will not necessarily suffer every side effect mentioned. If you have any questions or concerns, please ask your doctor, nurse or pharmacist or telephone one of the contact numbers on this information sheet. What is spironolactone? The liquid preparation comes in 5mg/ml, 10mg/5ml, 25mg/5ml, 50mg/5ml and 100mg/5ml Spironolactone belongs to a group of medicines strengths. You should use the oral syringe called diuretics. It is commonly used alongside provided to draw up the correct amount of another medicine called furosemide to reduce liquid and squirt it in the inside of your child’s fluid overload, so reducing the amount of work cheek. Please note that some of these strengths the heart has to do to pump blood around the may not be available locally so you should body. It works by making the kidneys produce always tell the pharmacist the strength of your more urine (wee), so your child may have to child’s usual medicine. go to the toilet more often. It also prevents potassium being lost in the urine. It is commonly Your doctor, nurse or pharmacist will have prescribed for children with heart or kidney explained to you how to obtain the correct problems causing excess fluid in the body. -

Pharmacological Interaction of Lopinavir
C S & lini ID ca A l f R o e l s Journal of Schmaltz et al., J AIDS Clin Res 2014, 5:10 a e n a r r c u h DOI: 10.4172/2155-6113.1000358 o J ISSN: 2155-6113 AIDS & Clinical Research Research Article Open Access Pharmacological Interaction of Lopinavir/Ritonavir 800/200 mg BID and Rifampicin in Subjects Presenting Tuberculosis with Contraindication for an Efavirenz containing Antiretroviral Regimen Carolina Arana Stanis Schmaltz1*, Marli Jane Martins Costa1, Vitória Berg Cattani1, Douglas Pereira Pinto1, José Liporage¹, Aline Benjamin1, Catherine Boulanger3, Mariza Morgado2 and Valeria Rolla1 1Institute of Clinical Research Evandro Chagas , Fiocruz , Brazil 2Institute of Oswaldo Cruz, Fiocruz, Brazil 3School of Medicine, Division of Infectious Disease, University of Miami, USA Abstract Rifampicin reduces plasma concentration of most HIV protease inhibitors. Lopinavir boosted with ritonavir (LPV/r) could be an option to treat TB-HIV patients. Our aim was to evaluate lopinavir interaction with rifampicin during TB-HIV therapy. TB-HIV patients who could not use efavirenz and with no genotypic resistance to lopinavir were included. Rifampicin 600 mg, isoniazid 400 mg and pyrazinamide 2000 mg were started at day one for 6 months and LPV/r plus two nucleoside/nucleotide reverse transcriptase inhibitors were introduced at day 30. LPV/r dose was started at 400/100 mg BID and escalated over 7 days to 800/200 mg BID. Pharmacokinetic sampling was performed at day 15 (rifampicin), 45, 90, 180 (rifampicin, lopinavir, ritonavir) and 210 (lopinavir, ritonavir). Viral load (VL) and CD4 counts were performed at baseline and days 30, 60, 120, and 180. -

NORVIR and Certain Other Drugs May Result in These Highlights Do Not Include All the Information Needed to Use Known Or Potentially Significant Drug Interactions
HIGHLIGHTS OF PRESCRIBING INFORMATION • The concomitant use of NORVIR and certain other drugs may result in These highlights do not include all the information needed to use known or potentially significant drug interactions. Consult the full NORVIR safely and effectively. See full prescribing information for prescribing information prior to and during treatment for potential drug NORVIR. interactions. (5.1, 7.2) • Hepatotoxicity: Fatalities have occurred. Monitor liver function before and NORVIR (ritonavir) capsules, soft gelatin for oral use during therapy, especially in patients with underlying hepatic disease, Initial U.S. Approval: 1996 including hepatitis B and hepatitis C, or marked transaminase elevations. (5.2, 8.6) WARNING: DRUG-DRUG INTERACTIONS LEADING TO • Pancreatitis: Fatalities have occurred; suspend therapy as clinically POTENTIALLY SERIOUS AND/OR LIFE THREATENING appropriate. (5.3) REACTIONS • Allergic Reactions/Hypersensitivity: Allergic reactions have been reported See full prescribing information for complete boxed warning and include anaphylaxis, toxic epidermal necrolysis, Stevens-Johnson Co-administration of NORVIR with several classes of drugs including syndrome, bronchospasm and angioedema. Discontinue treatment if severe sedative hypnotics, antiarrhythmics, or ergot alkaloid preparations may reactions develop. (5.4, 6.2) result in potentially serious and/or life-threatening adverse events due to • PR interval prolongation may occur in some patients. Cases of second and possible effects of NORVIR on the hepatic metabolism of certain drugs. third degree heart block have been reported. Use with caution with patients Review medications taken by patients prior to prescribing NORVIR or with preexisting conduction system disease, ischemic heart disease, when prescribing other medications to patients already taking NORVIR cardiomyopathy, underlying structural heart disease or when administering (4, 5.1) with other drugs that may prolong the PR interval. -

HIGHLIGHTS of PRESCRIBING INFORMATION Hepatotoxicity: Patients with Hepatitis B Or C Are at Risk of Increased Transaminases Or Hepatic Decompensation
HIGHLIGHTS OF PRESCRIBING INFORMATION Hepatotoxicity: Patients with hepatitis B or C are at risk of increased transaminases or hepatic decompensation. Monitor liver function tests These highlights do not include all the information needed to use prior to therapy and during treatment. (2.4, 5.5, 6.3, 6.4, 8.8) Atazanavir Sulfate and Ritonavir Tablets (300 mg/100 mg) safely and Nephrolithiasis has been reported. Consider temporary interruption or effectively. See full prescribing information for Atazanavir Sulfate and discontinuation. (5.6, 6.4) Ritonavir Tablets. Patients may develop new onset or exacerbations of diabetes mellitus/hyperglycemia (5.7, 6.3), immune reconstitution syndrome Atazanavir Sulfate and Ritonavir Tablets, 300 mg/100 mg (5.8), and redistribution/accumulation of body fat. (5.9) Hemophilia: Spontaneous bleeding may occur and additional factor VIII ------------------------INDICATIONS AND USAGE------------------------------- may be required. (5.10) Atazanavir Sulfate and Ritonavir Tablets, a combination of a protease Drug Interactions: Consider drug-drug interaction potential to reduce inhibitor and a protease inhibitor used as a pharmacokinetic enhancer, is risk of serious or life-threatening adverse reactions. (5.1) indicated for use in combination with other antiretroviral agents for the Pancreatitis: Fatalities have occurred; suspend therapy as clinically treatment of HIV-1 infection. (1) appropriate. (5.13) Total cholesterol and triglycerides elevations: Monitor prior to therapy -----------------------DOSAGE AND ADMINISTRATION----------------------- and periodically thereafter. (5.14) Take Atazanavir Sulfate and Ritonavir Tablets, 300 mg/100 mg, with food. Adults and pediatric patients (at least 6 years of age and 40 kg): ----------------------------ADVERSE REACTIONS--------------------------------- Atazanavir sulfate and Ritonavir Tablet, 300 mg/100 mg once daily with The most common adverse reactions (> 5%) were asthenia, malaise, anorexia, food.