RIFAMPICIN Productinformation Sigma Prod
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CLINICAL USE of RIFABUTIN, a RIFAMYCIN-CLASS ANTIBIOTIC, for the TREATMENT of TUBERCULOSIS (A Supplement to the 2008 Revision Of“ Standards for Tuberculosis Care”)
Kekkaku Vol. 86, No. 1: 43, 2011 43 CLINICAL USE OF RIFABUTIN, A RIFAMYCIN-CLASS ANTIBIOTIC, FOR THE TREATMENT OF TUBERCULOSIS (A supplement to the 2008 revision of“ Standards for tuberculosis care”) August, 2008 The Treatment Committee of the Japanese Society for Tuberculosis The Treatment Committee of the Japanese Society for [Dosage and administration of rifabutin] Tuberculosis published statements on the“ Standards for Rifabutin, 5 mg/kg in body weight/day, maximum 300 mg/ tuberculosis care” in April 2008. Therein we referred to day, once daily. rifampicin as follows“; Use of rifampicin requires attention The dosage of rifabutin can be increased up to the maximum because of the interactions with a number of other drugs. daily dose of 450 mg in cases where decreased rifabutin serum Particularly for HIV-infected patients who need antiviral levels are expected due to anti-HIV drugs such as efavirenz, drugs, the replacement of rifampicin by rifabutin should be and in other cases if necessary. considered”. Rifabutin, belonging to rifamycin-class antibiotics In non-HIV-infected patients, rifabutin can be used for like rifampicin, causes less significant drug-drug interactions intermittent treatment with a regimen of twice or three times a than rifampicin, and can be used in combination with antiviral week, with the same dosage as daily administration. drugs mentioned above. In July 2008, rifabutin was approved as antituberculous drug, and is expected to be added to the drug [Important points for use of rifabutin] price listing in the near future*. Therefore, to the published (1) Rifabutin causes drug interactions due to induction of opinions, we add new statements concerning the use of rifabutin hepatic enzyme though less significantly than rifampicin. -

PROCUR Why Procur Has Been Prescribed for You
Consumer Medicine Information Ask your doctor if you have any questions about PROCUR why Procur has been prescribed for you. Cyproterone acetate 50 mg and 100 mg tablets This medicine is available only with a doctor's prescription. What is in this leaflet Before you take Procur Please read this leaflet carefully before you start taking Procur When you must not take it This leaflet answers some common questions about Procur. It does not contain all the available Do not take Procur if you have an allergy to: information. It does not take the place of talking • any medicine containing cyproterone acetate to your doctor or pharmacist. • any of the ingredients listed at the end of this leaflet All medicines have risks and benefits. Your doctor has weighed the risks of you taking Procur against Some of the symptoms of an allergic reaction may the benefits they expect it will have for you. include: • difficulty in breathing or wheezing If you have any concerns about taking this • shortness of breath medicine, ask your doctor or pharmacist. • swelling of the face, tongue, lips, or other parts of the body Keep this leaflet with the medicine. You may • hives on the skin, rash, or itching need to read it again. Do not take Procur if: What Procur is used for • you are allergic to cyproterone acetate or any other ingredient listed at the end of this leaflet Procur tablets contain the active ingredient • you are pregnant cyproterone acetate. Cyproterone acetate is an • you are breastfeeding antiandrogen. It works by blocking the actions of • you suffer from liver diseases (including sex hormones (androgens) that are produced previous or existing liver tumours, Dubin- mainly in men but also, to a lesser extent in Johnson syndrome or Rotor syndrome) women. -

Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
pharmaceutics Review Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Luíse L. Chaves 1,2,*, Yuri Patriota 2, José L. Soares-Sobrinho 2 , Alexandre C. C. Vieira 1,3, Sofia A. Costa Lima 1,4 and Salette Reis 1,* 1 Laboratório Associado para a Química Verde, Rede de Química e Tecnologia, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, 4050-313 Porto, Portugal; [email protected] (A.C.C.V.); slima@ff.up.pt (S.A.C.L.) 2 Núcleo de Controle de Qualidade de Medicamentos e Correlatos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil; [email protected] (Y.P.); [email protected] (J.L.S.-S.) 3 Laboratório de Tecnologia dos Medicamentos, Universidade Federal de Pernambuco, Recife 50740-521, Brazil 4 Cooperativa de Ensino Superior Politécnico e Universitário, Instituto Universitário de Ciências da Saúde, 4585-116 Gandra, Portugal * Correspondence: [email protected] (L.L.C.); shreis@ff.up.pt (S.R.) Received: 30 October 2020; Accepted: 4 December 2020; Published: 11 December 2020 Abstract: Leprosy disease remains an important public health issue as it is still endemic in several countries. Mycobacterium leprae, the causative agent of leprosy, presents tropism for cells of the reticuloendothelial and peripheral nervous system. Current multidrug therapy consists of clofazimine, dapsone and rifampicin. Despite significant improvements in leprosy treatment, in most programs, successful completion of the therapy is still sub-optimal. Drug resistance has emerged in some countries. This review discusses the status of leprosy disease worldwide, providing information regarding infectious agents, clinical manifestations, diagnosis, actual treatment and future perspectives and strategies on targets for an efficient targeted delivery therapy. -

AMEG Categorisation of Antibiotics
12 December 2019 EMA/CVMP/CHMP/682198/2017 Committee for Medicinal Products for Veterinary use (CVMP) Committee for Medicinal Products for Human Use (CHMP) Categorisation of antibiotics in the European Union Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 29 October 2018 Adopted by the CVMP for release for consultation 24 January 2019 Adopted by the CHMP for release for consultation 31 January 2019 Start of public consultation 5 February 2019 End of consultation (deadline for comments) 30 April 2019 Agreed by the Antimicrobial Advice ad hoc Expert Group (AMEG) 19 November 2019 Adopted by the CVMP 5 December 2019 Adopted by the CHMP 12 December 2019 Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2020. Reproduction is authorised provided the source is acknowledged. Categorisation of antibiotics in the European Union Table of Contents 1. Summary assessment and recommendations .......................................... 3 2. Introduction ............................................................................................ 7 2.1. Background ........................................................................................................ -

Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis
Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Centers for Disease Control and Prevention Office of Infectious Diseases National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination June 2013 This document is accessible online at http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Suggested citation: CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis [online]. 2013. Available from URL: http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm Table of Contents Introduction 1 Methodology for Preparation of these Guidelines 2 The Role of Rifamycins in Tuberculosis Treatment 4 Managing Drug Interactions with Antivirals and Rifampin 5 Managing Drug Interactions with Antivirals and Rifabutin 9 Treatment of Latent TB Infection with Rifampin or Rifapentine 10 Treating Pregnant Women with Tuberculosis and HIV Co-infection 10 Treating Children with HIV-associated Tuberculosis 12 Co-treatment of Multidrug-resistant Tuberculosis and HIV 14 Limitations of these Guidelines 14 HIV-TB Drug Interaction Guideline Development Group 15 References 17 Table 1a. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in adults 21 Table 1b. Recommendations for regimens for the concomitant treatment of tuberculosis and HIV infection in children 22 Table 2a. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in adults 23 Table 2b. Recommendations for co-administering antiretroviral drugs with RIFAMPIN in children 25 Table 3. Recommendations for co-administering antiretroviral drugs with RIFABUTIN in adults 26 ii Introduction Worldwide, tuberculosis is the most common serious opportunistic infection among people with HIV infection. -
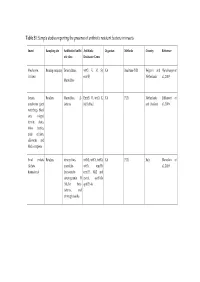
Table S1: Sample Studies Reporting the Presence of Antibiotic Resistant Bacteria in Insects
Table S1: Sample studies reporting the presence of antibiotic resistant bacteria in insects Insect Sampling site Antibiotics/Antibi Antibiotic Organism Methods Country Reference otic class Resistance Genes Mealworm, Rearing company Tetracyclines, tet(O, K, M, S), NA Real-time PCR Belgium and (Vandeweyer et Crickets erm(B) Netherlands al., 2019) Macrolides locusts, Retailers Macrolides; β- Erm(B, C), tet(O, K, NA PCR Netherlands (Milanović et mealworm giant lactams M, S), blaZ and Thailand al., 2016) waterbugs, black ants, winged termite alates, rhino beetles, mole crickets, silkworm, and black scorpions Small crickets Retailers tetracyclines, tet(M), tet(O), tet(K), NA PCR Italy (Roncolini et (Acheta macrolide- tet(S), erm(B), al., 2019) domesticus) lincosamide- erm(C), blaZ and streptogramin B mecA, aac(6')-Ie (MLSB), beta- aph(2")-Ia lactams, and aminoglycosides Cockroach Hospital Penicillins, blaZ, aacA-D, tetK, Staphylococcus Culture, PCR Iran (Abdolmaleki environment Cephems, msrA, dfrA, msrA, aureus et al., 2019) Aminoglycosides, vatB, tetM, grlA, macrolides. Folate rpoB, vatA, linA, inhibitors, ermA, cat1 Tetracyclines, fluoroquinolones, Lincosamides, Phenicols, Ansamycins Cockroaches Household ampicillin, NA Enterobacter spp., Culture Iran (Vazirianzadeh kitches cephalothin, Klebsiella et al., 2014) ceftriaxone, spp., Citrobacter ciproflexoxacin, spp., E. coli, chloramphenicol, Salmonella spp., gentamicin, Proteus spp., tetracycline, coagulase negative trimethoprim- staph- ylococci, S. sulfamethoxazole, marcescens, ceftazidime, -

MEPRON® (Atovaquone) Suspension
NDA 20-500/S-010 Page 3 PRESCRIBING INFORMATION MEPRON® (atovaquone) Suspension DESCRIPTION MEPRON (atovaquone) is an antiprotozoal agent. The chemical name of atovaquone is trans- 2-[4-(4-chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione. Atovaquone is a yellow crystalline solid that is practically insoluble in water. It has a molecular weight of 366.84 and the molecular formula C22H19ClO3. The compound has the following structural formula: MEPRON Suspension is a formulation of micro-fine particles of atovaquone. The atovaquone particles, reduced in size to facilitate absorption, are significantly smaller than those in the previously marketed tablet formulation. MEPRON Suspension is for oral administration and is bright yellow with a citrus flavor. Each teaspoonful (5 mL) contains 750 mg of atovaquone and the inactive ingredients benzyl alcohol, flavor, poloxamer 188, purified water, saccharin sodium, and xanthan gum. MICROBIOLOGY Mechanism of Action: Atovaquone is a hydroxy-1,4-naphthoquinone, an analog of ubiquinone, with antipneumocystis activity. The mechanism of action against Pneumocystis carinii has not been fully elucidated. In Plasmodium species, the site of action appears to be the cytochrome bc1 complex (Complex III). Several metabolic enzymes are linked to the mitochondrial electron transport chain via ubiquinone. Inhibition of electron transport by atovaquone will result in indirect inhibition of these enzymes. The ultimate metabolic effects of such blockade may include inhibition of nucleic acid and ATP synthesis. Activity In Vitro: Several laboratories, using different in vitro methodologies, have shown the IC50 (50% inhibitory concentration) of atovaquone against rat P. carinii to be in the range of 0.1 to 3.0 mcg/mL. -

Spironolactone
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Spironolactone This information sheet should be read in conjunction with any information provided by the manufacturer. This information sheet explains what spironolactone is, how it is given and some of the possible side effects. Each person reacts differently to medicines, so your child will not necessarily suffer every side effect mentioned. If you have any questions or concerns, please ask your doctor, nurse or pharmacist or telephone one of the contact numbers on this information sheet. What is spironolactone? The liquid preparation comes in 5mg/ml, 10mg/5ml, 25mg/5ml, 50mg/5ml and 100mg/5ml Spironolactone belongs to a group of medicines strengths. You should use the oral syringe called diuretics. It is commonly used alongside provided to draw up the correct amount of another medicine called furosemide to reduce liquid and squirt it in the inside of your child’s fluid overload, so reducing the amount of work cheek. Please note that some of these strengths the heart has to do to pump blood around the may not be available locally so you should body. It works by making the kidneys produce always tell the pharmacist the strength of your more urine (wee), so your child may have to child’s usual medicine. go to the toilet more often. It also prevents potassium being lost in the urine. It is commonly Your doctor, nurse or pharmacist will have prescribed for children with heart or kidney explained to you how to obtain the correct problems causing excess fluid in the body. -

Pharmacological Interaction of Lopinavir
C S & lini ID ca A l f R o e l s Journal of Schmaltz et al., J AIDS Clin Res 2014, 5:10 a e n a r r c u h DOI: 10.4172/2155-6113.1000358 o J ISSN: 2155-6113 AIDS & Clinical Research Research Article Open Access Pharmacological Interaction of Lopinavir/Ritonavir 800/200 mg BID and Rifampicin in Subjects Presenting Tuberculosis with Contraindication for an Efavirenz containing Antiretroviral Regimen Carolina Arana Stanis Schmaltz1*, Marli Jane Martins Costa1, Vitória Berg Cattani1, Douglas Pereira Pinto1, José Liporage¹, Aline Benjamin1, Catherine Boulanger3, Mariza Morgado2 and Valeria Rolla1 1Institute of Clinical Research Evandro Chagas , Fiocruz , Brazil 2Institute of Oswaldo Cruz, Fiocruz, Brazil 3School of Medicine, Division of Infectious Disease, University of Miami, USA Abstract Rifampicin reduces plasma concentration of most HIV protease inhibitors. Lopinavir boosted with ritonavir (LPV/r) could be an option to treat TB-HIV patients. Our aim was to evaluate lopinavir interaction with rifampicin during TB-HIV therapy. TB-HIV patients who could not use efavirenz and with no genotypic resistance to lopinavir were included. Rifampicin 600 mg, isoniazid 400 mg and pyrazinamide 2000 mg were started at day one for 6 months and LPV/r plus two nucleoside/nucleotide reverse transcriptase inhibitors were introduced at day 30. LPV/r dose was started at 400/100 mg BID and escalated over 7 days to 800/200 mg BID. Pharmacokinetic sampling was performed at day 15 (rifampicin), 45, 90, 180 (rifampicin, lopinavir, ritonavir) and 210 (lopinavir, ritonavir). Viral load (VL) and CD4 counts were performed at baseline and days 30, 60, 120, and 180. -

Short-Course Rifamycin-Based Regimens for TB Infection (LTBI): Why Countries Should Scale up This Silver Bullet for TB Prevention Among PLHIV
Short-course rifamycin-based regimens for TB infection (LTBI): Why countries should scale up this silver bullet for TB prevention among PLHIV TB/HIV Research Meeting organized by WHO In conjunction with CROI, Boston, MA, USA March 4, 2018 Presented by: Kelly Dooley MD, PhD Johns Hopkins University School of Medicine D I V I S I O N O F CLINICAL PHARMACOLOGY 1 Latent TB infection (LTBI) About 1 in 4 persons Houben and Dodd. PLoS Med 2016;13(10):e1002152 http://www.who.int/tb/challenges/ltbi_factsheet_25nov15.pdf?ua=1; 2 http://www.results.org.au/living-with-hiv-dying-of-tb/; https://msdh.ms.gov/msdhsite/_static/14,0,150,728.html Treatment of LTBI reduces risk of TB disease & death in patients with HIV infection, independent of ART 0.25 0.20 Did not start IPT Started IPT 0.15 0.10 Cumulative of probability tuberculosis 0.05 Risk of TB diseaseRisk Risk of death Risk 0.00 1 mo 1 yr 2 yr 3 yr 4 yr 5 yr 6 yr 7 yr Years since PPD+ Number at risk (events) Did not start IPT 1222 (58) 400 (14) 318 (9) 241 (1) 168 (2) 123 (2) 84 (0) 62 Started IPT 732 (7) 1470 (12) 1506 (12) 1437 (2) 1149 (5) 790 (3) 414 (0) 189 IPT for 6 months Temprano IPT for 6 months HIV+, TST+ , Rio de Janeiro ANRS Study HIV+, TST not done, Côte d’Ivoire 3 Golub et al CID 2015 Badje et al., Lancet Global Health, 2017 Not prescribed, not taken Completion rates varied from 6% to 94% “… and were inversely proportional to the duration of treatment” WHO 2018 Guidelines on the management of latent tuberculosis infection Fox et al 2017 IJID 4 Whither shorter-course rifamycin-based -

Strong Selective Agents Determine Resistance Evolution in a Multidrug
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.01.406181; this version posted December 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: Strong Selective Agents Determine Resistance Evolution in a Multidrug Therapeutic Regime Authors: Johannes Cairns1,2*, Florian Borse1, Tommi Mononen1, Teppo Hiltunen2,3*, Ville Mustonen1,4*. 5 Affiliations: 1Organismal and Evolutionary Biology Research Programme (OEB), Department of Computer Science, 00014 University of Helsinki, Helsinki, Finland. 2Department of Microbiology, 00014 University of Helsinki, Helsinki, Finland. 3Department of Biology, 20014 University of Turku, Turku, Finland. 10 4Helsinki Institute for Information Technology, Institute of Biotechnology, 00014 University of Helsinki, Helsinki, Finland. *Correspondence to: [email protected] or [email protected] or v.mustonen@hel- sinki.fi. ORCIDs: JC, 0000-0003-1329-2025; FB, 0000-0003-4232-257X; TM, 0000-0002-3603-0813; 15 TH, 0000-0001-7206-2399; VM, 0000-0002-7270-1792. Abstract: Multidrug regimes have been considered to constrain selection for resistance compared to monotherapy. However, drug resistance trajectories are influenced by a wide range of conditions which can cause opposing outcomes. Here we employed an in vitro model system to investigate differences in resistance dynamics between mono-, combination and alternating regimes. Across 20 regimes involving three drugs and phage, selection for resistance was decreased in multidrug re- gimes compared to monotherapy. Surprisingly, across regimes, two out of the four agents used to impose selection had a dominant effect on the overall outcome. Resistance to these agents either caused cross-resistance or obscured the phenotypic effect of other resistance mutations. -

Therapy of Rhodococcus Equi Infections in Foals
IN-DEPTH: RHODOCOCCUS EQUI Therapy of Rhodococcus equi Infections in Foals Steeve Gigue`re, DVM, PhD, Diplomate ACVIM The combination of a macrolide (erythromycin, azithromycin, or clarithromycin) with rifampin re- mains the mainstay of therapy. Although the vast majority of Rhodococcus equi isolates are still susceptible to these antimicrobials, the frequency of macrolide-rifampin–resistant isolates seems to be increasing. Author’s address: College of Veterinary Medicine, University of Georgia, Athens, Georgia 30602; e-mail: [email protected]. © 2010 AAEP. 1. Introduction lates are inhibited (MIC90) is 0.12, 0.25, and A wide variety of antimicrobial agents are active 1.0 g/ml for clarithromycin, erythromycin, and 5 against Rhodococcus equi in vitro. However, be- azithromycin, respectively. The combination of a cause R. equi is a facultative intracellular pathogen macrolide and rifampin is synergistic both in vitro surviving and replicating in macrophages, many of and in vivo, and the use of the two classes of drugs in these drugs are ineffective in vivo. For example, in combination reduces the likelihood of R. equi resis- one study, all 17 foals with R. equi pneumonia tance to either drug.4,6 Rifampin and macrolides treated with the combination of penicillin and gen- are lipid soluble, allowing them to penetrate cell tamicin died despite the fact that all isolates were membranes and caseous material. sensitive to gentamicin.1 The combination of ri- The recommended doses are listed in Table fampin and erythromycin became the treatment of 1. Several formulations of erythromycin are com- choice in the 1980s and has dramatically reduced mercially available.