DB 2020 42 4 Cuckoos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
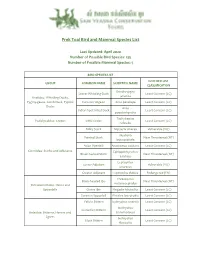
Prek Toal Bird and Mammal Species List
Prek Toal Bird and Mammal Species List Last Updated: April 2020 Number of Possible Bird Species: 135 Number of Possible Mammal Species: 5 BIRD SPECIES LIST IUCN RED LIST GROUP COMMON NAME SCIENTIFIC NAME CLASSIFICATION Dendrocygna Lesser Whistling-Duck Least Concern (LC) javanica Anatidae: Whistling Ducks, Pygmy-geese, Comb Duck, Typical Eurasian Wigeon Anas penelope Least Concern (LC) Ducks Anas Indian Spot-billed Duck Least Concern (LC) poecilorhyncha Tachybaptus Podicipedidae: Grebes Little Grebe Least Concern (LC) ruficollis Milky Stork Mycteria cinerea Vulnerable (VU) Mycteria Painted Stork Near Threatened (NT) leucocephala Asian Openbill Anastomus oscitans Least Concern (LC) Ciconiidae: Storks and Adjutants Ephippiorhynchus Black-necked Stork Near Threatened (NT) asiaticus Leptoptilos Lesser Adjutant Vulnerable (VU) javanicus Greater Adjutant Leptoptilos dubius Endangered (EN) Threskiornis Black-headed Ibis Near Threatened (NT) melanocephalus Threskiornithidae: Ibises and Spoonbills Glossy Ibis Plegadis falcinellus Least Concern (LC) Eurasian Spoonbill Platalea leucorodia Least Concern (LC) Yellow Bittern Ixobrychus sinensis Least Concern (LC) Ixobrychus Cinnamon Bittern Least Concern (LC) Ardeidae: Bitterns, Herons and cinnamomeus Egrets Ixobrychus Black Bittern Least Concern (LC) flavicollis Black-crowned Nycticorax Least Concern (LC) Night-Heron nycticorax Little Heron Butorides striata Least Concern (LC) Chinese Pond-Heron Ardeola bacchus Least Concern (LC) Javan Pond-Heron Ardeola speciosa Least Concern (LC) Bubulcus Eastern -

SICHUAN (Including Northern Yunnan)
Temminck’s Tragopan (all photos by Dave Farrow unless indicated otherwise) SICHUAN (Including Northern Yunnan) 16/19 MAY – 7 JUNE 2018 LEADER: DAVE FARROW The Birdquest tour to Sichuan this year was a great success, with a slightly altered itinerary to usual due to the closure of Jiuzhaigou, and we enjoyed a very smooth and enjoyable trip around the spectacular and endemic-rich mountain and plateau landscapes of this striking province. Gamebirds featured strongly with 14 species seen, the highlights of them including a male Temminck’s Tragopan grazing in the gloom, Chinese Monal trotting across high pastures, White Eared and Blue Eared Pheasants, Lady Amherst’s and Golden Pheasants, Chinese Grouse and Tibetan Partridge. Next were the Parrotbills, with Three-toed, Great and Golden, Grey-hooded and Fulvous charming us, Laughingthrushes included Red-winged, Buffy, Barred, Snowy-cheeked and Plain, we saw more Leaf Warblers than we knew what to do with, and marvelled at the gorgeous colours of Sharpe’s, Pink-rumped, Vinaceous, Three-banded and Red-fronted Rosefinches, the exciting Przevalski’s Finch, the red pulse of Firethroats plus the unreal blue of Grandala. Our bird of the trip? Well, there was that Red Panda that we watched for ages! 1 BirdQuest Tour Report: Sichuan Including Northern Yunnan 2018 www.birdquest-tours.com Our tour began with a short extension in Yunnan, based in Lijiang city, with the purpose of finding some of the local specialities including the rare White-speckled Laughingthrush, which survives here in small numbers. Once our small group had arrived in the bustling city of Lijiang we began our birding in an area of hills that had clearly been totally cleared of forest in the fairly recent past, with a few trees standing above the hillsides of scrub. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
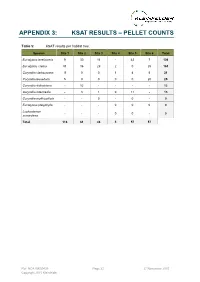
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential -

The Birds and Other Wildlife Recorded on the David Bishop Bird Tours Bhutan Tour - 2015
The Birds and Other Wildlife recorded on the David Bishop Bird Tours Bhutan Tour - 2015 Wallcreeper © K. David Bishop Compiled and led by K. David Bishop David Bishop Bird Tours Bhutan 2015 BHUTAN 2015 “The Paro Dzong (monastery), guarded by icy crags, sits warming under the late afternoon sun. It seems to welcome our approach to our beautifully located hotel. An Ibisbill, so subtle as to be taken for a glacial stone, dips quietly in the snowmelt. This is indeed the Kingdom of Bhutan and the land of the peaceful Dragon.” As my good friend Steve Hilty remarked on first setting foot in the kingdom, "This is fairytale land." K. David Bishop This was my 28th bird tour to Bhutan. I first began leading bird tours to this magical kingdom in 1994 and have enjoyed the privilege of returning there once or twice a year almost annually since then. So what is it that has makes this particular tour so attractive? Quite simply Bhutan is in a class of its very own. Yes it is an expensive tour (although with David Bishop Bird Tours perhaps not so), largely because the Bhutanese have decided (in our opinion quite rightly) that they would rather not compromise their culture and spectacular natural environment to hundreds of thousands of tourists and in consequence they charge a princely sum for being among the privileged few to visit their country. Similarly we feel that we have a very special product to offer and whilst we could make it shorter and thus less expensive we feel that that would diminish the experience. -

A Preliminary Survey of Bird Communities of South Sikkim, India
International Journal of Zoology Studies International Journal of Zoology Studies ISSN: 2455-7269; Impact Factor: RJIF 5.14 Received: 01-10-2020; Accepted: 15-10-2020; Published: 02-11-2020 www.zoologyjournals.com Volume 5; Issue 6; 2020; Page No. 04-10 A preliminary survey of bird communities of South Sikkim, India John Bhutia1, Pamina Chettri2, Bishnu K Sharma3 1, 2 Department of Zoology, Namchi Government College, Kamrang South Sikkim, India 3 Department of Botany, Namchi Government College, Kamrang South Sikkim, India Abstract Sikkim (27003’-28007’N & 88003’-88057’E), a small state lies on the foothills of Himalayas, comprising only of 7096 sq km, having a varied climatic condition and vegetation, ranging from cold desert in the north to the lowland forest in south. The study of avian faunal diversity is an essential ecological tool, which acts as an indicator to evaluated different habitats both qualitatively and quantitatively The present day study was carried out to documents the avian diversity in South District of Sikkim. A total of 100 species of birds belonging to 35 families were recorded. In terms of familial richness, muscicapidae dominates in the field of study areas, comprising 16 species followed by turdidae with 8 species, corvdae with 6 species. The present day study adds some valuable information on avian diversity in the study area. Keywords: avian fauna, birds, community, sikkim and conservation 1. Introduction The increase on the number of threatened species is mainly Birds are prominent species of global biodiversity because of habitat destruction and other anthropogenic (Olechnowski, 2009) [19] and key indicators of ecosystem factors. -

Himachal Wildlife Project Report
.. .. -.. - . ;.,.. ~ .... ~ ... '.' 4 . • . ~ .. : ': '. 'to.. _. .... ':~~' ." • ' .. " . / " .}t.. .. .. , ....."• • .. ." • ~ILDl:IF'~~"OF .HIMACHAL PRA"DES~ THE ~ •• . .. , _.-.,-- THE WILDLIFE OF HIMACHAL PRADESH WESTERN HIMALAYAS Report of the Himachal Wildlife Project 1981 Technical Notes No.B2 School of Forest Resources University of Maine Albert D. Nutting Hall Orono, Maine 04469 U.S.A. $5.00 Left to right CRB HLH AJG GK PCT VH rJe VJZ AJG SCS AHG RP RK AC VH JH SKC HLH PJC HHR GK PJG CONTRIBUTORS Dr. C. R. Babu, Dept. of Botany, University of Delhi S. K. Chattopadhyay, Zoological Survey of India, Calcutta A. Chauduri, Dept. of Botany , University of Delhi P. J. Cioffi, School of Forest Resources, University of Maine Dr . P. J. Garson , Dept. of Zoology, University of Newcastle-upon-Tyne Dr. A. J. Gaston, Canadian Wildlife Service, Ottawa Dr . M. L. Hunter, Jr., School of Forest Resources, University of Maine R. Khandwa, Dept. of Botany, University of Delhi A. Kitsos, Jr. , School of Forest Resources, University of Maine G. Kumar, Zoological Survey of India, Dehra Dun V. Hatthai, Bombay Natural History Society Ramakrishnan Palat, Dept. of Zoology, University of Cali cut P. R. Phillimore, Dept. of Anthropology, University of Durham H. H. Ridley, Dept. of Zoology, University of Oxford S. C. Sharma, Dept. of Zoology & Entomology, Palampur Agricultural University P. C. Tak, Zoological Survey of India, Dehra Dun B. Vashisht, Dept. of Botany. University of Delhi S. K. Vats, Palampur J. W. Witham, School of Forest Resources, University of Maine Dr. V. J. Zacharias, Dept. of Zoology, University of Calicut EDITORS &1thony J. Gaston, Canadian Wildlife Service, Ottawa, Ontario KIA OE7 Canada Malcolm L. -

Supporting References for Nelson & Ellis
Supplemental Data for Nelson & Ellis (2018) The citations below were used to create Figures 1 & 2 in Nelson, G., & Ellis, S. (2018). The History and Impact of Digitization and Digital Data Mobilization on Biodiversity Research. Publication title by year, author (at least one ADBC funded author or not), and data portal used. This list includes papers that cite the ADBC program, iDigBio, TCNs/PENs, or any of the data portals that received ADBC funds at some point. Publications were coded as "referencing" ADBC if the authors did not use portal data or resources; it includes publications where data was deposited or archived in the portal as well as those that mention ADBC initiatives. Scroll to the bottom of the document for a key regarding authors (e.g., TCNs) and portals. Citation Year Author Portal used Portal or ADBC Program was referenced, but data from the portal not used Acevedo-Charry, O. A., & Coral-Jaramillo, B. (2017). Annotations on the 2017 Other Vertnet; distribution of Doliornis remseni (Cotingidae ) and Buthraupis macaulaylibrary wetmorei (Thraupidae ). Colombian Ornithology, 16, eNB04-1 http://asociacioncolombianadeornitologia.org/wp- content/uploads/2017/11/1412.pdf [Accessed 4 Apr. 2018] Adams, A. J., Pessier, A. P., & Briggs, C. J. (2017). Rapid extirpation of a 2017 Other VertNet North American frog coincides with an increase in fungal pathogen prevalence: Historical analysis and implications for reintroduction. Ecology and Evolution, 7, (23), 10216-10232. Adams, R. P. (2017). Multiple evidences of past evolution are hidden in 2017 Other SEINet nrDNA of Juniperus arizonica and J. coahuilensis populations in the trans-Pecos, Texas region. -

Host Selection in Parasitic Birds: Are Open-Cup Nesting Insectivorous Passerines Always Suitable Cuckoo Hosts?
Journal of Avian Biology 44: 216–220, 2013 doi: 10.1111/j.1600-048X.2013.00123.x © 2013 Th e Authors. Journal of Avian Biology © 2013 Nordic Society Oikos Subject Editor: Ronald Ydenberg. Accepted 11 February 2013 Host selection in parasitic birds: are open-cup nesting insectivorous passerines always suitable cuckoo hosts? Canchao Yang , B å rd G. Stokke , Anton Antonov † , Yan Cai , Suhua Shi , Arne Moksnes , Eivin R ø skaft , Anders P. M ø ller , Wei Liang and Tom á š Grim W. Liang ([email protected]), Y. Cai and C. Yang, Ministry of Education Key Laboratory for Tropical Plant and Animal Ecology, College of Life Sciences, Hainan Normal Univ., CN-571158 Haikou, PR China. – B. G. Stokke, A. Antonov, A. Moksnes and E. R øskaft, Dept of Biology, Norwegian Univ. of Science and Technology, NO-7491 Trondheim, Norway. – S. Shi, State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen Univ., CN-510275 Guangzhou, PR China. – A. P. M ø ller, Laboratoire d’Ecologie, Syst é matique et Evolution, CNRS UMR 8079, Univ. Paris-Sud, FR-91405 Orsay Cedex, France. – T. Grim, Dept of Zoology and Laboratory of Ornithology, Palacky Univ., 17. listopadu 50, CZ-77146 Olomouc, Czech Republic. How do potential hosts escape detrimental interactions with brood parasites? Current consensus is that hole-nesting and granivorous birds avoid brood parasites, like common cuckoos Cuculus canorus , by their inaccessible nest-sites and food unsuitable for parasites, respectively. Any open-nesting insectivorous hosts are believed to remain open to brood parasite exploitation which leads to the evolution of costly host defences like egg or chick discrimination. -

Updated Peer-Review of the Wildlife Conservation Plan of the WII, Etalin Hydropower Project, Dibang, Arunachal Pradesh, 5 May 20
Peer-review of the Wildlife Conservation Plan, prepared by the Wildlife Institute of India (WII) for the Etalin Hydropower Project, Dibang Valley, Arunachal Pradesh 5 May 2020 CONTRIBUTORS LISTED ALPHABETICALLY Anindya Sinha, PhD, National Institute of Advanced Studies, Bengaluru Anirban Datta Roy, PhD, Independent researcher Arjun Kamdar, National Centre for Biological Sciences, Bengaluru Aparajita Datta, PhD, Senior Scientist, Nature Conservation Foundation, Bengaluru Chihi Umbrey, MSc, Department of Zoology, Rajiv Gandhi University, Itanagar, Arunachal Pradesh Chintan Sheth, MSc, Independent researcher M. Firoz Ahmed, PhD, Scientist F, Head, Herpetofauna Research and Conservation Division, Aaranyak, Guwahati Jagdish Krishnaswamy, PhD, Convenor and Senior Fellow, Ashoka Trust for Research in Ecology and the Environment, Bengaluru Jayanta Kumar Roy, PhD, Senior Researcher, Herpetofauna Research and Conservation Division, Aaranyak, Guwahati Karthik Teegalapalli, PhD, Independent researcher Khyanjeet Gogoi, TOSEHIM, Regional Orchids Germplasm Conservation and Propagation Centre, Assam Circle Krishnapriya Tamma, PhD, Azim Premji University, Bengaluru Manish Kumar, PhD, Fellow, Centre for Ecology Development and Research, Uttarakhand Megha Rao, MSc, Nature Conservation Foundation, Bengaluru Monsoonjyoti Gogoi, PhD, Scientist B, Bombay Natural History Society Narayan Sharma, PhD, Assistant Professor, Cotton University, Guwahati Neelesh Dahanukar, PhD, Scientist, Zoo Outreach Organization, Coimbatore Rajeev Raghavan, PhD, South Asia Coordinator, -

'The Devil Is in the Detail': Peer-Review of the Wildlife Conservation Plan By
‘The devil is in the detail’: Peer-review of the Wildlife Conservation Plan by the Wildlife Institute of India for the Etalin Hydropower Project, Dibang Valley Chintan Sheth1, M. Firoz Ahmed2*, Sayan Banerjee3, Neelesh Dahanukar4, Shashank Dalvi1, Aparajita Datta5, Anirban Datta Roy1, Khyanjeet Gogoi6, Monsoonjyoti Gogoi7, Shantanu Joshi8, Arjun Kamdar8, Jagdish Krishnaswamy9, Manish Kumar10, Rohan K. Menzies5, Sanjay Molur4, Shomita Mukherjee11, Rohit Naniwadekar5, Sahil Nijhawan1, Rajeev Raghavan12, Megha Rao5, Jayanta Kumar Roy2, Narayan Sharma13, Anindya Sinha3, Umesh Srinivasan14, Krishnapriya Tamma15, Chihi Umbrey16, Nandini Velho1, Ashwin Viswanathan5 & Rameshori Yumnam12 1Independent researcher, Ananda Nilaya, 4th Main Road, Kodigehalli, Bengaluru, Karnataka 560097, India Email: [email protected] (corresponding author) 2Herpetofauna Research and Conservation Division, Aaranyak, Guwahati, Assam. 3National Institute of Advanced Studies, Bengaluru, Karnataka. 4Zoo Outreach Organization, Coimbatore, Tamil Nadu. 5Nature Conservation Foundation, Bengaluru, Karnataka. 6TOSEHIM, Regional Orchids Germplasm Conservation and Propagation Centre, Assam Circle, Assam. 7Bombay Natural History Society, Mumbai, Maharashtra. 8National Centre for Biological Sciences, Bengaluru, Karnataka. 9Ashoka Trust for Research in Ecology and the Environment, Bengaluru, Karnataka. 10Centre for Ecology Development and Research, Uttarakhand. 11Sálim Ali Centre for Ornithology and Natural History (SACON), Coimbatore, Tamil Nadu. 12South Asia IUCN Freshwater Fish -

The Timor Leaf-Warbler Phylloscopus Presbytes Is a Probable Host of the Sunda Cuckoo Cuculus Lepidus
Kukila 14 2009 Short Communication 49 The Timor Leaf-Warbler Phylloscopus presbytes Is a Probable Host of the Sunda Cuckoo Cuculus lepidus MARK SCHELLEKENS Goirkestraat 146, 5046 GP Tilburg, The Netherlands (alternative address, Woloara – Ende – Kelimutu, Flores, N.T.T. 86372, Indonesia. Email: [email protected] Ringkasan. Tulisan ini menyajikan hasil kunjungan ke Ende, Flores Tengah tanggal 20 September 2005 yang kemudian menemukan sepasang Cikrak Timor Phyllopscopus presbytes yang sedang memberi makan anak Kangkok Sunda Cuculus lepidus. Informasi ini kemungkinan merupakan laporan pertama mengenai pengasuhan oleh inang terhadap anak Cuculus lepidus untuk kawasan Wallacea. On a visit to Mount Kelimutu (846’S – 12149E), Ende regency, Central Flores, Nusa Tenggara, on 20 September 2005, a pair of Timor Leaf-warblers Phyllopscopus presbytes was observed feeding a juvenile Sunda Cuckoo Cuculus lepidus (formerly considered conspecific with Oriental Cuckoo C. optatus and Himalayan Cuckoo C. saturatus; see King 2005, Payne 2005). The cuckoo was large with dark, mottled upperparts, and a light (creamy) coloured, barred breast and belly. The bill was tipped black, and the eye-ring and legs were yellow. Observations were made at an altitude of c. 1,580 m asl from the path leading up to the viewing-point on the mountain. The cuckoo was observed perched in the mid-story of a Casuarina tree, and was continuously fed by the pair of Leaf- warblers for a period of at least 10 min. Food was mainly small caterpillars. During the feeding the chick constantly shook its tail and wings but made no calls. After 10 min the young cuckoo flew off into the forest with the Leaf- warblers and was not seen again. -

Bird Checklists of the World Country Or Region: Malaysia
Avibase Page 1of 23 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Malaysia 2 Number of species: 799 3 Number of endemics: 14 4 Number of breeding endemics: 0 5 Number of introduced species: 17 6 Date last reviewed: 2020-03-19 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Malaysia. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=my [23/09/2021]. Make your observations count! Submit your data to ebird.