Host Selection in Parasitic Birds: Are Open-Cup Nesting Insectivorous Passerines Always Suitable Cuckoo Hosts?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NSS Bird Group Report – November 2019
NSS Bird Group Report – November 2019 By Geoff Lim, Alan Owyong (compiler), Tan Gim Cheong (ed.). November was spectacular, with the first record of two species – the Fairy Pitta and Shikra at the Central Catchment Nature Reserve; an Oriental Dwarf Kingfisher (the locally extinct rufous- backed subspecies), found inside a camera shop in the city; and, a rare Red-footed Booby at St John’s Island. Also, it was and has always been a great month to spot migrating raptors in southern Singapore. A Fairy’s Visitation in November The first Fairy Pitta discovered in Singapore on 8 Nov 2019 – photo by Francis Yap. On 8 November 2019, Francis Yap and Richard White were en route to Jelutong Tower, when the duo spotted a paler than usual pitta along the trail under the darkening morning sky as a storm threatened from Sumatra. When Francis managed to regain phone reception and were able to refer to other photos on the internet, the two confirmed that they had Singapore’s first record of the Fairy Pitta, Pitta nympha. Francis’ electrifying account can be accessed here. The Fairy Pitta stopped over for a week, with daily records from 8-13 November 2019. 1 The Fairy Pitta has been recognised as part of a superspecies comprising the Blue-winged Pitta, P. moluccensis, Mangrove Pitta, P. megarhyncha, and Indian Pitta, P. brachyura (Lambert & Woodcock, 1996:162), hence the superficial resemblance with one another. BirdLife has classified the species as Vulnerable, with key threats being habitat loss and conversion, as well as local trapping pressure (BirdLife, 2019). -
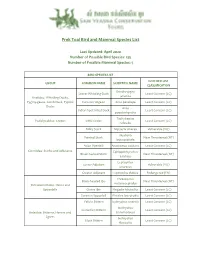
Prek Toal Bird and Mammal Species List
Prek Toal Bird and Mammal Species List Last Updated: April 2020 Number of Possible Bird Species: 135 Number of Possible Mammal Species: 5 BIRD SPECIES LIST IUCN RED LIST GROUP COMMON NAME SCIENTIFIC NAME CLASSIFICATION Dendrocygna Lesser Whistling-Duck Least Concern (LC) javanica Anatidae: Whistling Ducks, Pygmy-geese, Comb Duck, Typical Eurasian Wigeon Anas penelope Least Concern (LC) Ducks Anas Indian Spot-billed Duck Least Concern (LC) poecilorhyncha Tachybaptus Podicipedidae: Grebes Little Grebe Least Concern (LC) ruficollis Milky Stork Mycteria cinerea Vulnerable (VU) Mycteria Painted Stork Near Threatened (NT) leucocephala Asian Openbill Anastomus oscitans Least Concern (LC) Ciconiidae: Storks and Adjutants Ephippiorhynchus Black-necked Stork Near Threatened (NT) asiaticus Leptoptilos Lesser Adjutant Vulnerable (VU) javanicus Greater Adjutant Leptoptilos dubius Endangered (EN) Threskiornis Black-headed Ibis Near Threatened (NT) melanocephalus Threskiornithidae: Ibises and Spoonbills Glossy Ibis Plegadis falcinellus Least Concern (LC) Eurasian Spoonbill Platalea leucorodia Least Concern (LC) Yellow Bittern Ixobrychus sinensis Least Concern (LC) Ixobrychus Cinnamon Bittern Least Concern (LC) Ardeidae: Bitterns, Herons and cinnamomeus Egrets Ixobrychus Black Bittern Least Concern (LC) flavicollis Black-crowned Nycticorax Least Concern (LC) Night-Heron nycticorax Little Heron Butorides striata Least Concern (LC) Chinese Pond-Heron Ardeola bacchus Least Concern (LC) Javan Pond-Heron Ardeola speciosa Least Concern (LC) Bubulcus Eastern -

SICHUAN (Including Northern Yunnan)
Temminck’s Tragopan (all photos by Dave Farrow unless indicated otherwise) SICHUAN (Including Northern Yunnan) 16/19 MAY – 7 JUNE 2018 LEADER: DAVE FARROW The Birdquest tour to Sichuan this year was a great success, with a slightly altered itinerary to usual due to the closure of Jiuzhaigou, and we enjoyed a very smooth and enjoyable trip around the spectacular and endemic-rich mountain and plateau landscapes of this striking province. Gamebirds featured strongly with 14 species seen, the highlights of them including a male Temminck’s Tragopan grazing in the gloom, Chinese Monal trotting across high pastures, White Eared and Blue Eared Pheasants, Lady Amherst’s and Golden Pheasants, Chinese Grouse and Tibetan Partridge. Next were the Parrotbills, with Three-toed, Great and Golden, Grey-hooded and Fulvous charming us, Laughingthrushes included Red-winged, Buffy, Barred, Snowy-cheeked and Plain, we saw more Leaf Warblers than we knew what to do with, and marvelled at the gorgeous colours of Sharpe’s, Pink-rumped, Vinaceous, Three-banded and Red-fronted Rosefinches, the exciting Przevalski’s Finch, the red pulse of Firethroats plus the unreal blue of Grandala. Our bird of the trip? Well, there was that Red Panda that we watched for ages! 1 BirdQuest Tour Report: Sichuan Including Northern Yunnan 2018 www.birdquest-tours.com Our tour began with a short extension in Yunnan, based in Lijiang city, with the purpose of finding some of the local specialities including the rare White-speckled Laughingthrush, which survives here in small numbers. Once our small group had arrived in the bustling city of Lijiang we began our birding in an area of hills that had clearly been totally cleared of forest in the fairly recent past, with a few trees standing above the hillsides of scrub. -

K Here for the Full Trip Report
Capped Langur , Small Pratincole , WhiteWhite----wingedwinged Ducks , Gaur , Sultan Tit , Great Hornbill andand Pied Falconet ; Nameri Here a couple of ducks had now turned up , and while we were watching them , a Gaur suddenly came out of the forest for a drink and some fresh grass from the meadow surrounding the lake. We also saw several Wild Boars and a small group of Northern Red Muntjacs here , not to mention a Great Hornbill , Sultan Tits and a small party of Scarlet Minivets. No doubt this was a fantastic place , and there is no telling what could have been seen if more time had been spent here. Back in the camp we enjoyed yet another good meal , before driving a bit up river where this afternoons boat ride was to begin. We didn’t really see all that many birds while rafting on the river , but even so it was a nice experience to watch the beautiful landscape pass by in a leisurely pace – not exactly white water rafting this! Of course , there were a few avian highlights as well , including lots of Small Pratincoles , a couple of Crested Kingfisher and some nice River Lapwings , but we somehow managed to dip out on Ibisbill. We walked back to the camp as the sun was setting , but didn’t add anything new to our list , though a couple of Brown Hawk Owls put on quite a show for Erling , who was the first one to get back. We tried again with some spotlighting in the evening , and heard a calling Oriental Scops Owl not to far from the road but still impossible to see. -

Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Erforschung biologischer Ressourcen der Mongolei Institut für Biologie der Martin-Luther-Universität / Exploration into the Biological Resources of Halle-Wittenberg Mongolia, ISSN 0440-1298 2005 Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia Tobias Stenzel Martin-Luther-Universität, [email protected] Michael Stubbe Martin-Luther-Universität, [email protected] R. Samjaa National University of Mongolia, [email protected] S. Gombobaatar National University of Mongolia Follow this and additional works at: http://digitalcommons.unl.edu/biolmongol Part of the Asian Studies Commons, Biodiversity Commons, Desert Ecology Commons, Environmental Sciences Commons, Nature and Society Relations Commons, Ornithology Commons, Other Animal Sciences Commons, Other Ecology and Evolutionary Biology Commons, Population Biology Commons, and the Zoology Commons Stenzel, Tobias; Stubbe, Michael; Samjaa, R.; and Gombobaatar, S., "Quantitative Investigations on Bird Communities in Different Habitats in the Orkhon-Selenge-Valley in Northern Mongolia" (2005). Erforschung biologischer Ressourcen der Mongolei / Exploration into the Biological Resources of Mongolia, ISSN 0440-1298. 127. http://digitalcommons.unl.edu/biolmongol/127 This Article is brought to you for free and open access by the Institut für Biologie der Martin-Luther-Universität Halle-Wittenberg at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Erforschung biologischer Ressourcen der Mongolei / Exploration into the Biological Resources of Mongolia, ISSN 0440-1298 by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. In: Proceedings of the symposium ”Ecosystem Research in the Arid Environments of Central Asia: Results, Challenges, and Perspectives,” Ulaanbaatar, Mongolia, June 23-24, 2004. -

Žƶƌŷăů ŽĨ Dśƌğăƚğŷğě Dădžă
KWE^^ ůůĂƌƟĐůĞƐƉƵďůŝƐŚĞĚŝŶƚŚĞ:ŽƵƌŶĂůŽĨdŚƌĞĂƚĞŶĞĚdĂdžĂĂƌĞƌĞŐŝƐƚĞƌĞĚƵŶĚĞƌƌĞĂƟǀĞŽŵŵŽŶƐƩƌŝďƵƟŽŶϰ͘Ϭ/ŶƚĞƌŶĂͲ ƟŽŶĂů>ŝĐĞŶƐĞƵŶůĞƐƐŽƚŚĞƌǁŝƐĞŵĞŶƟŽŶĞĚ͘:ŽddĂůůŽǁƐƵŶƌĞƐƚƌŝĐƚĞĚƵƐĞŽĨĂƌƟĐůĞƐŝŶĂŶLJŵĞĚŝƵŵ͕ƌĞƉƌŽĚƵĐƟŽŶĂŶĚ ĚŝƐƚƌŝďƵƟŽŶďLJƉƌŽǀŝĚŝŶŐĂĚĞƋƵĂƚĞĐƌĞĚŝƚƚŽƚŚĞĂƵƚŚŽƌƐĂŶĚƚŚĞƐŽƵƌĐĞŽĨƉƵďůŝĐĂƟŽŶ͘ :ŽƵƌŶĂůŽĨdŚƌĞĂƚĞŶĞĚdĂdžĂ dŚĞŝŶƚĞƌŶĂƟŽŶĂůũŽƵƌŶĂůŽĨĐŽŶƐĞƌǀĂƟŽŶĂŶĚƚĂdžŽŶŽŵLJ ǁǁǁ͘ƚŚƌĞĂƚĞŶĞĚƚĂdžĂ͘ŽƌŐ /^^EϬϵϳϰͲϳϵϬϳ;KŶůŝŶĞͿͮ/^^EϬϵϳϰͲϳϴϵϯ;WƌŝŶƚͿ ÊÃÃçÄ®ã®ÊÄ ò®¥çÄÊ¥«Ã®ÝãÙ®ã͕,®Ã«½WÙÝ«͕/Ä®ó®ã« ÃÖ«Ý®ÝÊÄ<½ãÊÖͲ<«¹¹®Ùt®½½®¥^ÄãçÙùÄ®ãÝ ÝçÙÙÊçÄ®Ä¦Ý dĂƌŝƋŚŵĞĚ^ŚĂŚ͕sŝƐŚĂůŚƵũĂ͕DĂƌƟŶĂŶĂŶĚĂŵΘŚĞůŵĂůĂ ^ƌŝŶŝǀĂƐƵůƵ Ϯϲ:ĂŶƵĂƌLJϮϬϭϲͮsŽů͘ϴͮEŽ͘ϭͮWƉ͘ϴϯϯϯʹϴϯϱϳ ϭϬ͘ϭϭϲϬϵͬũŽƩ͘ϭϳϳϰ͘ϴ͘ϭ͘ϴϯϯϯͲϴϯϱϳ &Žƌ&ŽĐƵƐ͕^ĐŽƉĞ͕ŝŵƐ͕WŽůŝĐŝĞƐĂŶĚ'ƵŝĚĞůŝŶĞƐǀŝƐŝƚŚƩƉ͗ͬͬƚŚƌĞĂƚĞŶĞĚƚĂdžĂ͘ŽƌŐͬďŽƵƚͺ:Ždd͘ĂƐƉ &ŽƌƌƟĐůĞ^ƵďŵŝƐƐŝŽŶ'ƵŝĚĞůŝŶĞƐǀŝƐŝƚŚƩƉ͗ͬͬƚŚƌĞĂƚĞŶĞĚƚĂdžĂ͘ŽƌŐͬ^ƵďŵŝƐƐŝŽŶͺ'ƵŝĚĞůŝŶĞƐ͘ĂƐƉ &ŽƌWŽůŝĐŝĞƐĂŐĂŝŶƐƚ^ĐŝĞŶƟĮĐDŝƐĐŽŶĚƵĐƚǀŝƐŝƚ ŚƩƉ͗ͬͬƚŚƌĞĂƚĞŶĞĚƚĂdžĂ͘ŽƌŐͬ:ŽddͺWŽůŝĐLJͺĂŐĂŝŶƐƚͺ^ĐŝĞŶƟĮĐͺDŝƐĐŽŶĚƵĐƚ͘ĂƐƉ &ŽƌƌĞƉƌŝŶƚƐĐŽŶƚĂĐƚфŝŶĨŽΛƚŚƌĞĂƚĞŶĞĚƚĂdžĂ͘ŽƌŐх WƵďůŝƐŚĞƌͬ,ŽƐƚ WĂƌƚŶĞƌ dŚƌĞĂƚĞŶĞĚTaxa Journal of Threatened Taxa | www.threatenedtaxa.org | 26 January 2016 | 8(1): 8333–8357 Avifauna of Chamba District, Himachal Pradesh, India with emphasis on Kalatop-Khajjiar Wildlife Sanctuary and its Communication surroundings ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) Tariq Ahmed Shah 1, Vishal Ahuja 2, Martina Anandam 3 & Chelmala Srinivasulu 4 OPEN ACCESS 1,2,3 Field Research Division, Wildlife Information Liaison Development (WILD) Society, 96 Kumudham Nagar, Vilankurichi Road, Coimbatore, Tamil Nadu 641035, India 1,4 Natural History Museum and Wildlife -

Bhutan II Th Th 16 April to 5 May 2015 (20 Days)
Trip Report Bhutan II th th 16 April to 5 May 2015 (20 days) Ibisbill by Wayne Jones Trip report compiled by tour leader Wayne Jones Trip Report - RBT Bhutan II 2015 2 Our Bhutan tour kicked off at 350m above sea level in Samdrup Jongkhar, the border town close to Assam. The town's quiet gentility was quite a contrast to the hubbub of the Indian province in which we had just spent the last five days. Our arrival was in the late afternoon, so after settling into our hotel and meeting for dinner there wasn't much scope for birding. After supper, attempts to draw in a calling Collared Scops Owl were not entertained by the bird in question and a thunderstorm gently encouraged us to head to our rooms. This was to be the first of many encounters with rain in Bhutan! Crimson Sunbird by Wayne Jones The next morning we began our birding day with a walk along the main road on the outskirts of town while our bus went ahead to collect us later, the general modus operandi of birding in Bhutan. We glimpsed Red Junglefowl, Striated and Indian Pond Herons, Crested Honey Buzzard – one of which perched in a tree for good views, a Black Eagle cruising low over the treetops, Crested Goshawk, Green-billed Malkoha, House Swift, Wreathed Hornbill, Oriental Dollarbird, Lesser Yellownape, White-throated Kingfisher, Black-winged Cuckooshrike, Scarlet Minivet, Long-tailed Shrike, Ashy and Bronzed Drongos, Black-crested Bulbul, Red-rumped Swallow, Greenish Warbler, Rufescent Prinia, a gorgeous Asian Fairy-bluebird, a fleeting White-rumped Shama, common but beautiful Verditer Flycatcher, Black-backed Forktail, Blue Whistling Thrush, White- capped Redstart, Crimson Sunbird, Streaked Spiderhunter and Chestnut-tailed Starling. -

Thailand Custom Tour 29 January -13 February, 2017
Tropical Birding Trip Report THAILAND JANUARY-FEBRUARY, 2017 Thailand custom tour 29 January -13 February, 2017 TOUR LEADER: Charley Hesse Report by Charley Hesse. Photos by Charley Hesse & Laurie Ross. All photos were taken on this tour When it comes to vacation destinations, Thailand has it all: great lodgings, delicious food, scenery, good roads, safety, value for money and friendly people. In addition to both its quantity & quality of birds, it is also one of the most rapidly evolving destinations for bird photography. There are of course perennial favourite locations that always produce quality birds, but year on year, Thailand comes up with more and more fantastic sites for bird photography. On this custom tour, we followed the tried and tested set departure itinerary and found an impressive 420 species of birds and 16 species of mammals. Some of the highlights included: Spoon-billed Sandpiper and Nordmann’s Greenshank around Pak Thale; Wreathed Hornbill, Long-tailed & Banded Broadbills inside Kaeng Krachan National Park; Rosy, Daurian & Spot-winged Starlings at a roost site just outside; Kalij Pheasant, Scaly-breasted & Bar-backed Partridges at a private photography blind nearby; Siamese Fireback and Great Hornbill plus Asian Elephant & Malayan Porcupine at Khao Yai National Park; countless water birds at Bueng Boraphet; a myriad of montane birds at Doi Inthanon; Giant Nuthatch at Doi Chiang Dao; Scarlet-faced Liocichla at Doi Ang Khang; Hume’s Pheasant & Spot-breasted Parrotbill at Doi Lang; Yellow-breasted Buntings at Baan Thaton; and Baikal Bush-Warbler & Ferruginous Duck at Chiang Saen. It was a truly unforgettable trip. www.tropicalbirding.com +1-409-515-9110 [email protected] Tropical Birding Trip Report THAILAND JANUARY-FEBRUARY, 2017 29th January – Bangkok to Laem Pak Bia After a morning arrival in Bangkok, we left the sprawling metropolis on the overhead highways, and soon had our first birding stop at the Khok Kham area of Samut Sakhon, the neighbouring city to Bangkok. -

The Birds of the Wenyu
The Birds of the Wenyu Beijing’s Mother River Steve Bale 史進 1 Contents Introduction Page 3 The Status, The Seasons, The Months Page 9 The Birds Page 10 Finding Birds on the Wenyu Page 172 The List of the ‘New’ Birds for the Wenyu Page 178 Special Thanks Page 186 Free to Share… Page 187 References Page 188 2 Introduction In the meeting of the Zoological Society of London on the 22nd November 1842, John Gould (1804-81) presented what was described in the Society’s proceedings as a “new species of Parrot” 1. The impressively marked bird had been collected on the Marquesas Islands – a remote spot of the Pacific Ocean that would become part of French Polynesia. The members of the Society present at that meeting would have undoubtedly been impressed by yet another of the rare, exotic gems that Gould had a habit of pulling out of his seemingly bottomless hat. Next up in this Victorian frontiers-of-ornithology ‘show and tell’ was Hugh Edwin Strickland (1811-53). The birds he spoke about2 were quite a bit closer to home, although many were every bit as exotic as Gould’s Polynesian parrot. Strickland, instead of sourcing his specimens from the far corners of the Earth, had simply popped across London to Hyde Park Corner with his note book. There, causing quite a stir, was an exhibition of "Ten Thousand Chinese Things", displayed in a purpose-built “summer house” whose design was, according to The Illustrated London News3, “usual in the gardens of the wealthy, in the southern provinces of China”. -

Declines in Common and Migratory Breeding Landbird Species in South Korea Over the Past Two Decades
fevo-09-627765 March 22, 2021 Time: 13:49 # 1 ORIGINAL RESEARCH published: 29 March 2021 doi: 10.3389/fevo.2021.627765 Declines in Common and Migratory Breeding Landbird Species in South Korea Over the Past Two Decades Hankyu Kim1,2*†, Yongwon Mo3†, Chang-Yong Choi4†, Brenda C. McComb1,2† and Matthew G. Betts1,2† 1 Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, United States, 2 Forest Biodiversity Research Network, Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, United States, 3 Department of Landscape Architecture, Yeungnam University, Gyeongsan, South Korea, 4 Department of Agriculture, Edited by: Forestry and Bioresources, College of Agriculture and Life Sciences, Seoul National University, Seoul, South Korea Cagan H. Sekercioglu, The University of Utah, United States Reviewed by: Population declines in terrestrial bird species have been reported across temperate Richard A. Fuller, regions in the world and are attributed to habitat loss, climate change, or other direct The University of Queensland, Australia mortality sources. North American and European studies indicate that long-distance Richard Gregory, migrants, common species, and species associated with grasslands and agricultural University College London, United Kingdom lands are declining at the greatest rates. However, data from East Asia on avian *Correspondence: population trends and associated drivers are extremely sparse. We modeled changes Hankyu Kim in occupancy of 52 common breeding landbird species in South Korea between 1997– [email protected] 2005 and 2013–2019. Thirty-eight percent of the species showed evidence of declines, † ORCID: and seven of these were declining severely (46–95%). -

Red List of Bangladesh 2015
Red List of Bangladesh Volume 1: Summary Chief National Technical Expert Mohammad Ali Reza Khan Technical Coordinator Mohammad Shahad Mahabub Chowdhury IUCN, International Union for Conservation of Nature Bangladesh Country Office 2015 i The designation of geographical entitles in this book and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN, International Union for Conservation of Nature concerning the legal status of any country, territory, administration, or concerning the delimitation of its frontiers or boundaries. The biodiversity database and views expressed in this publication are not necessarily reflect those of IUCN, Bangladesh Forest Department and The World Bank. This publication has been made possible because of the funding received from The World Bank through Bangladesh Forest Department to implement the subproject entitled ‘Updating Species Red List of Bangladesh’ under the ‘Strengthening Regional Cooperation for Wildlife Protection (SRCWP)’ Project. Published by: IUCN Bangladesh Country Office Copyright: © 2015 Bangladesh Forest Department and IUCN, International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holders, provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holders. Citation: Of this volume IUCN Bangladesh. 2015. Red List of Bangladesh Volume 1: Summary. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, pp. xvi+122. ISBN: 978-984-34-0733-7 Publication Assistant: Sheikh Asaduzzaman Design and Printed by: Progressive Printers Pvt. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
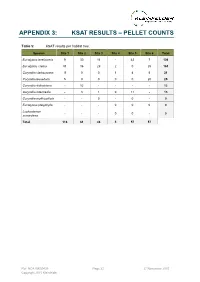
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential