Previously Unknown Behavior in Parasitic Cuckoo Females: Male-Like
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Importance of Muraviovka Park, Amur Province, Far East Russia, For
FORKTAIL 33 (2017): 81–87 The importance of Muraviovka Park, Amur province, Far East Russia, for bird species threatened at regional, national and international level based on observations between 2011 and 2016 WIELAND HEIM & SERGEI M. SMIRENSKI The middle reaches of the Amur River in Far East Russia are still an under-surveyed region, yet holding a very high regional biodiversity. During a six-year survey at Muraviovka Park, a non-governmental nature reserve, 271 bird species have been recorded, 14 of which are globally threatened, highlighting the importance of this area for bird conservation. INTRODUCTION RESULTS Recent studies have shown that East Asia and especially the Amur A total of 271 species was recorded inside Muraviovka Park between basin hold huge numbers of endangered species, and the region was 2011 and 2016; 24 species are listed as Near Treatened (NT), designated as a hotspot of threatened biodiversity (e.g. Vignieri 2014). Vulnerable (VU), Endangered (EN) or Critically Endangered (CR) Tis is especially true for birds. Te East Asian–Australasian Flyway (BirdLife International 2017a), 31 species in the Russian Red Data is not only one of the richest in species and individuals but is also the Book (Iliashenko & Iliashenko 2000) (Ru) and 60 species in the least surveyed and most threatened fyway (Yong et al. 2015). Current Amur region Red Data Book (Glushchenko et al. 2009) (Am). In data about distribution, population size and phenology are virtually the case of the Russian and Amur regional Red Data Books, the lacking for many regions, including the Amur region, Far East Russia. -

Madagascar, 1998
A mammal, bird, reptile, orchid and people-watching trip to - Madagascar (and a very short stay in Mauritius) 18-10-98 to 21-11-98 Dave Siems and Steve Anyon-Smith “weird (verb) – Madagascar” ------------------------------------------------------------------------------------------------------------ When our first guide, Patrice Rabearisoa, asked us what we wanted to see, he went white (not easy) at our reply – “we want to see all the birds, mammals, reptiles, orchids and everything else of interest in the forest, in no particular order.” He showed us all these things and more in the paradise that was, and still is, in parts, Madagascar. Outline of Trip “Madagascar” I said to Dave, and his eyes lit up. Five weeks later we were looking at lemurs. Our advice was that there was no safe or even practical way to visit a country populated by thieves, thugs and other human detritus of the worst order. There was said to be no usable public transport and if the food or the locals didn’t kill you, the insects most definitely would. So Dave and I set out to test these propositions. Madagascar is renowned for its wildlife, political instability and not much else. Our mission was to see as much of the native fauna and flora as possible during a five-week stay. We used public transport at all times and hired local guides at every location (this is generally compulsory anyway). We scattered ourselves widely throughout the country as the habitats are extremely varied, boasting rainforest, semi-desert, the so-called spiny forest and anything in between. Our expectations for the trip were not high given that we had little prior information and fully expected to be roasted slowly over a kitchen fire somewhere if we had managed to avoid perishing in a traffic accident. -

Bird Records from Laos, October 1994-August 1995
FORKTAIL 13 (1998): 33-68 Bird records from Laos, October 1994-August 1995 J. W. DUCKWORTH, R. J. TIZARD, R. J. TIMMINS, R. M. THEWLIS, W. G. ROBICHAUD and T. D. EVANS Between October 1994 and June 1995 birds were surveyed at six main areas in Laos, with incidental observations at many other sites extending until August 1995. Most effort was at four sites between 17°26' Nand 18°40' N (the Nakay Plateau, Phou Khaokhoay National Biodiversity Conservation Area (NBCA), Nam Kading NBCA and three nearby limestone outcrops), but there was extensive work on the Bolaven Plateau to the south and a brief visit to Phou Dendin NBCA in the extreme north. The latter is the first bird survey of a site much north of Vientiane since 1950. Information was collected for 15 Globally Threatened species and 28 Globally Near- Threatened species (sensu Collar et al. 1994), nine species At Risk in Laos, seven potentially so and one where threats in Laos are little known (sensu Thewlis et aL in prep.). A further Globally Near-Threatened species was recorded provisionally. Records of Grey-sided Thrush Turdus ftae and Black-headed Bunting Emberiza melanocephala were the first for Indochina. Ruddy Shelduck Tadorna ftrruginea, Lesser Cuckoo Cuculus poliocephalus,Pallas's Gull Larus ichthyaetus (from December 1995), Dunlin Calidris alpina, Long-toed Stint C subminutaand Chestnut-vented Nuthatch Sitta nagaensiswere new to Laos and Eurasian Blackbird Turdus merula to Cambodia. A total of 12 (plus one provisionally identified), nine and five (plus one provisionally identified) species were found new for North, Central and South Laos respectively. -
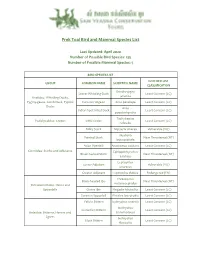
Prek Toal Bird and Mammal Species List
Prek Toal Bird and Mammal Species List Last Updated: April 2020 Number of Possible Bird Species: 135 Number of Possible Mammal Species: 5 BIRD SPECIES LIST IUCN RED LIST GROUP COMMON NAME SCIENTIFIC NAME CLASSIFICATION Dendrocygna Lesser Whistling-Duck Least Concern (LC) javanica Anatidae: Whistling Ducks, Pygmy-geese, Comb Duck, Typical Eurasian Wigeon Anas penelope Least Concern (LC) Ducks Anas Indian Spot-billed Duck Least Concern (LC) poecilorhyncha Tachybaptus Podicipedidae: Grebes Little Grebe Least Concern (LC) ruficollis Milky Stork Mycteria cinerea Vulnerable (VU) Mycteria Painted Stork Near Threatened (NT) leucocephala Asian Openbill Anastomus oscitans Least Concern (LC) Ciconiidae: Storks and Adjutants Ephippiorhynchus Black-necked Stork Near Threatened (NT) asiaticus Leptoptilos Lesser Adjutant Vulnerable (VU) javanicus Greater Adjutant Leptoptilos dubius Endangered (EN) Threskiornis Black-headed Ibis Near Threatened (NT) melanocephalus Threskiornithidae: Ibises and Spoonbills Glossy Ibis Plegadis falcinellus Least Concern (LC) Eurasian Spoonbill Platalea leucorodia Least Concern (LC) Yellow Bittern Ixobrychus sinensis Least Concern (LC) Ixobrychus Cinnamon Bittern Least Concern (LC) Ardeidae: Bitterns, Herons and cinnamomeus Egrets Ixobrychus Black Bittern Least Concern (LC) flavicollis Black-crowned Nycticorax Least Concern (LC) Night-Heron nycticorax Little Heron Butorides striata Least Concern (LC) Chinese Pond-Heron Ardeola bacchus Least Concern (LC) Javan Pond-Heron Ardeola speciosa Least Concern (LC) Bubulcus Eastern -

Assessment and Conservation of Threatened Bird Species at Laojunshan, Sichuan, China
CLP Report Assessment and conservation of threatened bird species at Laojunshan, Sichuan, China Submitted by Jie Wang Institute of Zoology, Chinese Academy of Sciences, Beijing, P.R.China E-mail:[email protected] To Conservation Leadership Programme, UK Contents 1. Summary 2. Study area 3. Avian fauna and conservation status of threatened bird species 4. Habitat analysis 5. Ecological assessment and community education 6. Outputs 7. Main references 8. Acknowledgements 1. Summary Laojunshan Nature Reserve is located at Yibin city, Sichuan province, south China. It belongs to eastern part of Liangshan mountains and is among the twenty-five hotspots of global biodiversity conservation. The local virgin alpine subtropical deciduous forests are abundant, which are actually rare at the same latitudes and harbor a tremendous diversity of plant and animal species. It is listed as a Global 200 ecoregion (WWF), an Important Bird Area (No. CN205), and an Endemic Bird Area (No. D14) (Stattersfield, et al . 1998). However, as a nature reserve newly built in 1999, it is only county-level and has no financial support from the central government. Especially, it is quite lack of scientific research, for example, the avifauna still remains unexplored except for some observations from bird watchers. Furthermore, the local community is extremely poor and facing modern development pressures, unmanaged human activities might seriously disturb the local ecosystem. We conducted our project from April to June 2007, funded by Conservation Leadership Programme. Two fieldwork strategies were used: “En bloc-Assessment” to produce an avifauna census and ecological assessments; "Special Survey" to assess the conservation status of some threatened endemic bird species. -

SICHUAN (Including Northern Yunnan)
Temminck’s Tragopan (all photos by Dave Farrow unless indicated otherwise) SICHUAN (Including Northern Yunnan) 16/19 MAY – 7 JUNE 2018 LEADER: DAVE FARROW The Birdquest tour to Sichuan this year was a great success, with a slightly altered itinerary to usual due to the closure of Jiuzhaigou, and we enjoyed a very smooth and enjoyable trip around the spectacular and endemic-rich mountain and plateau landscapes of this striking province. Gamebirds featured strongly with 14 species seen, the highlights of them including a male Temminck’s Tragopan grazing in the gloom, Chinese Monal trotting across high pastures, White Eared and Blue Eared Pheasants, Lady Amherst’s and Golden Pheasants, Chinese Grouse and Tibetan Partridge. Next were the Parrotbills, with Three-toed, Great and Golden, Grey-hooded and Fulvous charming us, Laughingthrushes included Red-winged, Buffy, Barred, Snowy-cheeked and Plain, we saw more Leaf Warblers than we knew what to do with, and marvelled at the gorgeous colours of Sharpe’s, Pink-rumped, Vinaceous, Three-banded and Red-fronted Rosefinches, the exciting Przevalski’s Finch, the red pulse of Firethroats plus the unreal blue of Grandala. Our bird of the trip? Well, there was that Red Panda that we watched for ages! 1 BirdQuest Tour Report: Sichuan Including Northern Yunnan 2018 www.birdquest-tours.com Our tour began with a short extension in Yunnan, based in Lijiang city, with the purpose of finding some of the local specialities including the rare White-speckled Laughingthrush, which survives here in small numbers. Once our small group had arrived in the bustling city of Lijiang we began our birding in an area of hills that had clearly been totally cleared of forest in the fairly recent past, with a few trees standing above the hillsides of scrub. -

Investment Cooperation
YENISEY SIBERIA DEVELOPMENT CORPORATION INVESTMENT PROPOSALS CONTENTS 3 About Yenisey Siberia Development Corporation 5 Investment proposals of Krasnoyarsk region 7 Development of building lime production 9 Modernization of amorphous graphite production 11 Troitsk saltworks ABOUT 13 Prime meridian medical centre 15 Latta antibacterial sprays YENISEY SIBERIA 17 Football arena chain 19 Marketplace coworking Street for eat DEVELOPMENT 21 Production of freeze-dried berries and functional beverages CORPORATION 23 Establishment of Uyar oil refinery 25 Kuznetsovo Glamping 27 Terephthalic acid production 29 Investment proposals of Khakassia republic 31 Fruit and berry garden 33 Pervomaysky dairy production complex Yenisey Siberia Development Corporation 35 Production of gypsum-based construction materials does not only provide comprehensive support for 37 Tasty Day ready-to-eat healthy food delivery chain large-scale projects but also supports promising investment projects. 39 Berkuty territory of river and cruise tourism 41 Priiskovy tourist and recreation facility development The investment proposals are promising projects 43 Podnebesye all-season resort with a mature concept, which initiators are already on the way of implementing their own ideas. Being at 45 IT center establishment the scaling stage, they are considering cooperation 49 Investment proposals of Tyva republic with a strategic investor as one of the financing options. 51 Full-service medical centre in Kyzyl 53 Full-service dental polyclinic in Kyzyl Interested investors are offered -

RCN #33 21/8/03 13:57 Page 1
RCN #33 21/8/03 13:57 Page 1 No. 33 Summer 2003 Special issue: The Transformation of Protected Areas in Russia A Ten-Year Review PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA RCN #33 21/8/03 13:57 Page 2 CONTENTS CONTENTS Voice from the Wild (Letter from the Editors)......................................1 Ten Years of Teaching and Learning in Bolshaya Kokshaga Zapovednik ...............................................................24 BY WAY OF AN INTRODUCTION The Formation of Regional Associations A Brief History of Modern Russian Nature Reserves..........................2 of Protected Areas........................................................................................................27 A Glossary of Russian Protected Areas...........................................................3 The Growth of Regional Nature Protection: A Case Study from the Orlovskaya Oblast ..............................................29 THE PAST TEN YEARS: Making Friends beyond Boundaries.............................................................30 TRENDS AND CASE STUDIES A Spotlight on Kerzhensky Zapovednik...................................................32 Geographic Development ........................................................................................5 Ecotourism in Protected Areas: Problems and Possibilities......34 Legal Developments in Nature Protection.................................................7 A LOOK TO THE FUTURE Financing Zapovedniks ...........................................................................................10 -

Bird Checklists of the World Country Or Region: Myanmar
Avibase Page 1of 30 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Myanmar 2 Number of species: 1088 3 Number of endemics: 5 4 Number of breeding endemics: 0 5 Number of introduced species: 1 6 7 8 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Myanmar. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=mm [23/09/2021]. Make your observations count! Submit your data to ebird. -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Rules
21282 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Rules and Regulations DEPARTMENT OF THE INTERIOR United States and the Government of United States or U.S. territories as a Canada Amending the 1916 Convention result of recent taxonomic changes; Fish and Wildlife Service between the United Kingdom and the (8) Change the common (English) United States of America for the names of 43 species to conform to 50 CFR Part 10 Protection of Migratory Birds, Sen. accepted use; and (9) Change the scientific names of 135 [Docket No. FWS–HQ–MB–2018–0047; Treaty Doc. 104–28 (December 14, FXMB 12320900000//201//FF09M29000] 1995); species to conform to accepted use. (2) Mexico: Convention between the The List of Migratory Birds (50 CFR RIN 1018–BC67 United States and Mexico for the 10.13) was last revised on November 1, Protection of Migratory Birds and Game 2013 (78 FR 65844). The amendments in General Provisions; Revised List of this rule were necessitated by nine Migratory Birds Mammals, February 7, 1936, 50 Stat. 1311 (T.S. No. 912), as amended by published supplements to the 7th (1998) AGENCY: Fish and Wildlife Service, Protocol with Mexico amending edition of the American Ornithologists’ Interior. Convention for Protection of Migratory Union (AOU, now recognized as the American Ornithological Society (AOS)) ACTION: Final rule. Birds and Game Mammals, Sen. Treaty Doc. 105–26 (May 5, 1997); Check-list of North American Birds (AOU 2011, AOU 2012, AOU 2013, SUMMARY: We, the U.S. Fish and (3) Japan: Convention between the AOU 2014, AOU 2015, AOU 2016, AOS Wildlife Service (Service), revise the Government of the United States of 2017, AOS 2018, and AOS 2019) and List of Migratory Birds protected by the America and the Government of Japan the 2017 publication of the Clements Migratory Bird Treaty Act (MBTA) by for the Protection of Migratory Birds and Checklist of Birds of the World both adding and removing species. -

Declines in Common and Migratory Breeding Landbird Species in South Korea Over the Past Two Decades
fevo-09-627765 March 22, 2021 Time: 13:49 # 1 ORIGINAL RESEARCH published: 29 March 2021 doi: 10.3389/fevo.2021.627765 Declines in Common and Migratory Breeding Landbird Species in South Korea Over the Past Two Decades Hankyu Kim1,2*†, Yongwon Mo3†, Chang-Yong Choi4†, Brenda C. McComb1,2† and Matthew G. Betts1,2† 1 Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, United States, 2 Forest Biodiversity Research Network, Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, United States, 3 Department of Landscape Architecture, Yeungnam University, Gyeongsan, South Korea, 4 Department of Agriculture, Edited by: Forestry and Bioresources, College of Agriculture and Life Sciences, Seoul National University, Seoul, South Korea Cagan H. Sekercioglu, The University of Utah, United States Reviewed by: Population declines in terrestrial bird species have been reported across temperate Richard A. Fuller, regions in the world and are attributed to habitat loss, climate change, or other direct The University of Queensland, Australia mortality sources. North American and European studies indicate that long-distance Richard Gregory, migrants, common species, and species associated with grasslands and agricultural University College London, United Kingdom lands are declining at the greatest rates. However, data from East Asia on avian *Correspondence: population trends and associated drivers are extremely sparse. We modeled changes Hankyu Kim in occupancy of 52 common breeding landbird species in South Korea between 1997– [email protected] 2005 and 2013–2019. Thirty-eight percent of the species showed evidence of declines, † ORCID: and seven of these were declining severely (46–95%). -

DIVERSITY of BIRDS ACROSS LAND USE and HABITAT GRADIENTS in FORESTS, RUBBER AGROFORESTS and RUBBER PLANTATIONS of NORTH SUMATRA Asep Ayat1,* and Hesti L
Indonesian Journal of Forestry Research Vol. 2, No. 2, October 2015, 103-120 ISSN: 2355-7079 / E-ISSN: 2406-8195 DIVERSITY OF BIRDS ACROSS LAND USE AND HABITAT GRADIENTS IN FORESTS, RUBBER AGROFORESTS AND RUBBER PLANTATIONS OF NORTH SUMATRA Asep Ayat1,* and Hesti L. Tata2 1Burung Indonesia, Jalan Dadali 32, Bogor 16161, Indonesia 2Forest Research and Development Center, Jl. Gunung Batu 5, Bogor, Indonesia Received: 31 March 2014, Revised: 10 May 2014, Accepted: 11 October 2015 DIVERSITY OF BIRDS ACROSS LAND USE AND HABITAT GRADIENTS IN FORESTS, RUBBER AGROFORESTS AND RUBBER PLANTATIONS OF NORTH SUMATRA. Birds play a pivotal role in the ecosystem, but in disturbed areas their roles may be limited due to the changes of their natural habitats. This paper studies the birds' habitats in Simalungun and Asahan Districts, North Sumatra. The study was conducted in four habitats: natural forest, rubber agroforests, rubber monoculture plantations and emplacement areas. The birds were observed using descriptive survey methods by implementing a quick biodiversity survey, data were collected along one km transect. The results showed that in total, 142 species of birds from 42 families were observed in the four habitats. Natural forests had the highest diversity of bird species, followed by rubber agroforests, emplacement areas and rubber plantations, with a Shannon-Wiener index of 3.8, 3.6, 3.0 and 2.9, respectively. Regarding the IUCN red list species, 12 bird species of near- threatened status and 2 species of vulnerable status were recorded. Based on CITES categories, one species was listed in the Appendix I, 12 species were classified in Appendix II and 26 bird species were protected under Indonesian regulations.