Fee Schedule Instructions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
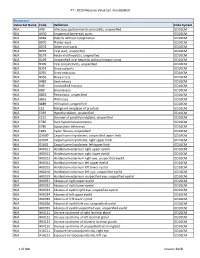
PT - 2020 Measure Value Set Avoidableed
PT - 2020 Measure Value Set_AvoidableED Numerator Value Set Name Code Definition Code System N/A A09 Infectious gastroenteritis and colitis, unspecified ICD10CM N/A A630 Anogenital (venereal) warts ICD10CM N/A B069 Rubella without complication ICD10CM N/A B070 Plantar wart ICD10CM N/A B078 Other viral warts ICD10CM N/A B079 Viral wart, unspecified ICD10CM N/A B179 Acute viral hepatitis, unspecified ICD10CM N/A B199 Unspecified viral hepatitis without hepatic coma ICD10CM N/A B309 Viral conjunctivitis, unspecified ICD10CM N/A B354 Tinea corporis ICD10CM N/A B355 Tinea imbricata ICD10CM N/A B356 Tinea cruris ICD10CM N/A B483 Geotrichosis ICD10CM N/A B49 Unspecified mycosis ICD10CM N/A B80 Enterobiasis ICD10CM N/A B852 Pediculosis, unspecified ICD10CM N/A B853 Phthiriasis ICD10CM N/A B889 Infestation, unspecified ICD10CM N/A C61 Malignant neoplasm of prostate ICD10CM N/A E039 Hypothyroidism, unspecified ICD10CM N/A E215 Disorder of parathyroid gland, unspecified ICD10CM N/A E780 Pure hypercholesterolemia ICD10CM N/A E786 Lipoprotein deficiency ICD10CM N/A E849 Cystic fibrosis, unspecified ICD10CM N/A G5600 Carpal tunnel syndrome, unspecified upper limb ICD10CM N/A G5601 Carpal tunnel syndrome, right upper limb ICD10CM N/A G5602 Carpal tunnel syndrome, left upper limb ICD10CM N/A H00011 Hordeolum externum right upper eyelid ICD10CM N/A H00012 Hordeolum externum right lower eyelid ICD10CM N/A H00013 Hordeolum externum right eye, unspecified eyelid ICD10CM N/A H00014 Hordeolum externum left upper eyelid ICD10CM N/A H00015 Hordeolum externum -

Rwanda ICD-10 Diagnostic Code Subset
Rwanda ICD-10 Diagnostic Code Subset ICD 10 code Diagnosis/Description A Infectious and parasitic diseases A00.0 CHOLERA DUE TO VIBRIO CHOLERAE 01_ BIOVAR CHOLERAE A01 TYPHOID AND PARATYPHOID FEVERS A01.0 TYPHOID FEVER A01.4 PARATYPHOID FEVER_ UNSPECIFIED A02 OTHER SALMONELLA INFECTIONS A02.0 SALMONELLA GASTROENTERITIS A02.1 SALMONELLA SEPTICEMIA A02.2 LOCALIZED SALMONELLA INFECTIONS A02.9 SALMONELLA INFECTION_ UNSPECIFIED A03 SHIGELLOSIS A03.0 SHIGELLOSIS DUE TO SHIGELLA DYSENTERIAE A03.2 SHIGELLOSIS DUE TO SHIGELLA BOYDII A03.9 SHIGELLOSIS_ UNSPECIFIED A04 OTHER BACTERIAL INTESTINAL INFECTIONS A04.0 ENTEROPATHOGENIC ESCHERICHIA COLI INFECTION A04.1 ENTEROTOXIGENIC ESCHERICHIA COLI INFECTION A04.4 OTHER INTESTINAL ESCHERICHIA COLI INFECTIONS A04.5 CAMPYLOBACTER ENTERITIS A04.7 ENTEROCOLITIS DUE TO CLOSTRIDIUM DIFFICILE A04.8 OTHER SPECIFIED BACTERIAL INTESTINAL INFECTIONS A04.9 BACTERIAL INTESTINAL INFECTION_ UNSPECIFIED A05 OTHER BACTERIAL FOOD-BORNE INTOXICATIONS A05.0 FOOD-BORNE STAPHYLOCOCCAL INTOXICATION A05.1 BOTULISM A05.4 FOOD-BORNE BACILLUS CEREUS INTOXICATION A05.8 OTHER SPECIFIED BACTERIAL FOOD-BORNE INTOXICATIONS A05.9 BACTERIAL FOOD-BORNE INTOXICATION_ UNSPECIFIED A06 AMEBIASIS A06.0 ACUTE AMEBIC DYSENTERY A06.1 CHRONIC INTESTINAL AMEBIASIS A06.2 AMEBIC NONDYSENTERIC COLITIS A06.3 AMEBOMA OF INTESTINE A06.4 AMEBIC LIVER ABSCESS A06.5 AMEBIC LUNG ABSCESS A06.6 AMEBIC BRAIN ABSCESS A06.7 CUTANEOUS AMEBIASIS A06.8 AMEBIC INFECTION OF OTHER SITES A06.9 AMEBIASIS_ UNSPECIFIED A07.0 BALANTIDIASIS A07.1 GIARDIASIS [LAMBLIASIS] -

(12) Patent Application Publication (10) Pub. No.: US 2016/0038462 A1 ZHANG Et Al
US 20160038462A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/0038462 A1 ZHANG et al. (43) Pub. Date: Feb. 11, 2016 (54) USE OF NK-1 RECEPTOR ANTAGONISTS IN (60) Provisional application No. 61/838,784, filed on Jun. PRURTUS 24, 2013. (71) Applicant: TIGERCAT PHARMA, INC., South Publication Classification San Francisco, CA (US) (51) Int. Cl. (72) Inventors: Xiaoming ZHANG, Sunnyvale, CA A613 L/403 (2006.01) (US); Edward F. SCHNIPPER, A6II 45/06 (2006.01) Redwood City, CA (US); Andrew J. A619/00 (2006.01) PERLMAN, Stanford, CA (US); James (52) U.S. Cl. W. LARRICK, Sunnyvale, CA (US) CPC ............. A6 IK3I/403 (2013.01); A61 K9/0053 (2013.01); A61 K9/0014 (2013.01); A61 K (21) Appl. No.: 14/922,684 45/06 (2013.01) (57) ABSTRACT (22)22) Filed: Oct. 26,9 2015 The invention relates to methods for treatingg pruritusp with NK-1 receptor antagonists such as serlopitant. The invention O O further relates to pharmaceutical compositions comprising Related U.S. Application Data NK-1 receptorantagonists such as serlopitant. In addition, the (60) Division of application No. 14/312.942, filed on Jun. invention encompasses treatment of a pruritus-associated 24, 2014, now Pat. No. 9,198.898, which is a continu condition with serlopitant and an additional antipruritic ation-in-part of application No. 13/925,509, filed on agent, and the use of serlopitant as a sleep aid, optionally in Jun. 24, 2013, now Pat. No. 8,906,951. combination with an additional sleep-aiding agent. Patent Application Publication Feb. -

190.18 - Serum Iron Studies
Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) 190.18 - Serum Iron Studies HCPCS Codes (Alphanumeric, CPT AMA) Code Description 82728 Ferritin 83540 Iron 83550 Iron Binding capacity 84466 Transferrin ICD-10-CM Codes Covered by Medicare Program The ICD-10-CM codes in the table below can be viewed on CMS’ website as part of Downloads: Lab Code List, at http://www.cms.gov/Medicare/Coverage/CoverageGenInfo/LabNCDsICD10.html Code Description A01.00 Typhoid fever, unspecified A01.01 Typhoid meningitis A01.02 Typhoid fever with heart involvement A01.03 Typhoid pneumonia A01.04 Typhoid arthritis A01.05 Typhoid osteomyelitis A01.09 Typhoid fever with other complications A01.1 Paratyphoid fever A A01.2 Paratyphoid fever B A01.3 Paratyphoid fever C A01.4 Paratyphoid fever, unspecified A02.0 Salmonella enteritis A02.1 Salmonella sepsis A02.20 Localized salmonella infection, unspecified NCD 190.18 January 2021 Changes ICD-10-CM Version – Red Fu Associates, Ltd. January 2021 1 Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) Code Description A02.21 Salmonella meningitis A02.22 Salmonella pneumonia A02.23 Salmonella arthritis A02.24 Salmonella osteomyelitis A02.25 Salmonella pyelonephritis A02.29 Salmonella with other localized infection A02.8 Other specified salmonella infections A02.9 Salmonella infection, unspecified A04.0 Enteropathogenic Escherichia coli infection A04.1 Enterotoxigenic Escherichia coli infection A04.2 Enteroinvasive Escherichia -

The Clinical Conundrum of Pruritus Victoria Garcia-Albea, Karen Limaye
FEATURE ARTICLE The Clinical Conundrum of Pruritus Victoria Garcia-Albea, Karen Limaye ABSTRACT: Pruritus is a common complaint for derma- Pruritus is also the most common symptom in derma- tology patients. Diagnosing the cause of pruritus can tological disease and can be a symptom of several sys- be difficult and is often frustrating for patients and pro- temic diseases (Bernhard, 1994). The overall incidence viders. Even after the diagnosis is made, it can be a chal- of pruritus is unknown because there are no epidemio- lenge to manage and relieve pruritus. This article reviews logical databases for pruritus (Norman, 2003). According common and uncommon causes of pruritus and makes to a 2003 study, pruritus and xerosis are the most com- recommendations for proper and thorough evaluation mon dermatological problems encountered in nursing and management. home patients (Norman, 2003). Key words: Itch, Pruritus INTRODUCTION ETIOLOGY Pruritus (itch) is the most frequent symptom in derma- The skin is equipped with a network of afferent sensory tology (Serling, Leslie, & Maurer, 2011). Itch is the pre- and efferent autonomic nerve branches that respond to dominant symptom associated with acute and chronic various chemical mediators found in the skin (Bernhard, cutaneous disease and is a major symptom in systemic dis- 1994). It is believed that maximal itch production is ease (Elmariah & Lerner, 2011; Steinhoff, Cevikbas, Ikoma, achieved at the basal layer of the epidermis, below which & Berger, 2011). It can be a frustration for both the patient pain is perceived (Bernhard, 1994). Autonomic nerves in- and the clinician. This article will attempt to provide an nervate hair follicles, pili erector muscles, blood vessels, overview of pruritus, a method for thorough and com- eccrine, apocrine, and sebaceous glands (Bernhard, 1994). -

Blood Glucose Testing 2020.Pdf
National Coverage Determination Procedure Code: 82947, 82948, 82962 Blood Glucose Testing CMS Policy Number: 190.20 See also: Medicare Preventive Services Back to NCD List Description: This policy is intended to apply to blood samples used to determine glucose levels. Blood glucose determination may be done using whole blood, serum or plasma. It may be sampled by capillary puncture, as in the fingerstick method, or by vein puncture or arterial sampling. The method for assay may be by color comparison of an indicator stick, by meter assay of whole blood or a filtrate of whole blood, using a device approved for home monitoring, or by using a laboratory assay system using serum or plasma. The convenience of the meter or stick color method allows a patient to have access to blood glucose values in less than a minute or so and has become a standard of care for control of blood glucose, even in the inpatient setting. Indications: Blood glucose values are often necessary for the management of patients with diabetes mellitus, where hyperglycemia and hypoglycemia are often present. They are also critical in the determination of control of blood glucose levels in patient with impaired fasting glucose (FPG 110-125 mg/dL), patient with insulin resistance syndrome and/or carbohydrate intolerance (excessive rise in glucose following ingestion of glucose/glucose sources of food), in patient with a hypoglycemia disorder such as nesidioblastosis or insulinoma, and in patients with a catabolic or malnutrition state. In addition to conditions listed, glucose testing may be medically necessary in patients with tuberculosis, unexplained chronic or recurrent infections, alcoholism, coronary artery disease (especially in women), or unexplained skin conditions (i.e.: pruritis, skin infections, ulceration and gangrene without cause). -

Read Code Description 14L.. H/O: Drug Allergy 158.. H/O: Abnormal Uterine Bleeding 16C2
Read Code Description 14L.. H/O: drug allergy 158.. H/O: abnormal uterine bleeding 16C2. Backache 191.. Tooth symptoms 191Z. Tooth symptom NOS 1927. Dry mouth 198.. Nausea 199.. Vomiting 19C.. Constipation 1A23. Incontinence of urine 1A32. Cannot pass urine - retention 1B1G. Headache 1B62. Syncope/vasovagal faint 1B75. Loss of vision 1BA2. Generalised headache 1BA3. Unilateral headache 1BA4. Bilateral headache 1BA5. Frontal headache 1BA6. Occipital headache 1BA7. Parietal headache 1BA8. Temporal headache 1C13. Deafness 1C131 Unilateral deafness 1C132 Partial deafness 1C133 Bilateral deafness 1C14. "Blocked ear" 1C15. Popping sensation in ear 1C1Z. Hearing symptom NOS 22J.. O/E - dead 22J4. O/E - dead - sudden death 22L4. O/E - Wound infected 2542. O/E - dental caries 2554. O/E - gums - blue line 2555. O/E - hypertrophy of gums 2FF.. O/E - skin ulcer 2I14. O/E - a rash 39C0. Pressure sore 39C1. Superficial pressure sore 39C2. Deep pressure sore 62... Patient pregnant 6332. Single stillbirth 66G4. Allergy drug side effect 72001 Enucleation of eyeball 7443. Exteriorisation of trachea 744D. Tracheo-oesophageal puncture 7511. Surgical removal of tooth 75141 Root canal therapy to tooth 7610. Total excision of stomach 7645. Creation of ileostomy 773C. Other operations on bowel 773Cz Other operation on bowel NOS 7826. Incision of bile duct 7840. Total excision of spleen 7B01. Total nephrectomy 7C032 Unilateral total orchidectomy - unspecified 7E117 Left salpingoophorectomy 7E118 Right salpingectomy 7E119 Left salpingectomy 7G321 Avulsion of nail 7H220 Exploratory laparotomy 7J174 Manipulation of mandible 8HG.. Died in hospital 94B.. Cause of death A.... Infectious and parasitic diseases A0... Intestinal infectious diseases A00.. Cholera A000. -
2019 Texas FI PA Results Ready for Formatting 3-29-20
2019 Texas Fully Insured Pharmacy Prior Authorization Requests Summary Prior Prior Total % Prior % Prior Appeal Appeal Total % Appeals % Appeals IRO Denials IRO Denials Total % IRO % IRO Authorization Authorization Prior Authorizations Authorizations Denials Denials Appeals Upheld Overturned Upheld Overturned IRO Upheld Overturned Approvals Denials Authorizations Approved Denied Upheld Overturned 23,211 7,514 30,725 76% 24% 235 293 528 45% 55% 7 1 8 88% 13% #Proprietary 1 of 83 2019 Texas Pharmacy Prior Authorization Report by Drug Name and 6-digit GPI Drug Name 1st 6 Prior Prior Total Prior % Prior % Prior Appeal Appeal Total Appeals % Appeals % Appeals IRO Denials IRO Denials Total IRO % IRO % IRO digits of Authorization Authorization Authorizations Authorizations Authorizations Denials Denials Upheld Overturned Upheld Overturned Upheld Overturned GPI Approval Denial Approved Denied Upheld Overturned 1STMEDX-PATC 908599 2 2 0% 100% ABILIFY 592500 6 4 10 60% 40% ABIRATERONE 214060 6 6 100% 0% ABSORICA 900500 47 26 73 64% 36% 1 1 2 50% 50% ACANYA 900599 1 1 2 50% 50% ACCU-CHEK 941000 3 2 5 60% 40% ACETAMINOPHEN CAFFEINE 659913 3 2 5 60% 40% ACETAMINOPHEN CODEINE 659910 192 16 208 92% 8% ACETAZOLAMIDE 371000 9 5 14 64% 36% 2 2 0% 100% ACETAZOLAMIDE 611000 2 2 100% 0% ACIPHEX 492700 6 4 10 60% 40% ACITRETIN 902505 1 1 100% 0% ACTEMRA 665000 17 9 26 65% 35% 2 2 0% 100% ACTICLATE 665000 1 1 100% 0% ACTONEL 300420 1 1 100% 0% ACTOPLUS 279980 1 1 100% 0% ACZONE 900510 248 11 259 96% 4% ADAPALENE 900500 11 2 13 85% 15% ADAPALENE 900599 13 5 -

Icd-10Causeofdeath.Pdf
A00 Cholera A00.0 Cholera due to Vibrio cholerae 01, biovar cholerae A00.1 Cholera due to Vibrio cholerae 01, biovar el tor A00.9 Cholera, unspecified A01 Typhoid and paratyphoid fevers A01.0 Typhoid fever A01.1 Paratyphoid fever A A01.2 Paratyphoid fever B A01.3 Paratyphoid fever C A01.4 Paratyphoid fever, unspecified A02 Other salmonella infections A02.0 Salmonella gastroenteritis A02.1 Salmonella septicemia A02.2 Localized salmonella infections A02.8 Other specified salmonella infections A02.9 Salmonella infection, unspecified A03 Shigellosis A03.0 Shigellosis due to Shigella dysenteriae A03.1 Shigellosis due to Shigella flexneri A03.2 Shigellosis due to Shigella boydii A03.3 Shigellosis due to Shigella sonnei A03.8 Other shigellosis A03.9 Shigellosis, unspecified A04 Other bacterial intestinal infections A04.0 Enteropathogenic Escherichia coli infection A04.1 Enterotoxigenic Escherichia coli infection A04.2 Enteroinvasive Escherichia coli infection A04.3 Enterohemorrhagic Escherichia coli infection A04.4 Other intestinal Escherichia coli infections A04.5 Campylobacter enteritis A04.6 Enteritis due to Yersinia enterocolitica A04.7 Enterocolitis due to Clostridium difficile A04.8 Other specified bacterial intestinal infections A04.9 Bacterial intestinal infection, unspecified A05 Other bacterial food-borne intoxications A05.0 Food-borne staphylococcal intoxication A05.1 Botulism A05.2 Food-borne Clostridium perfringens [Clostridium welchii] intoxication A05.3 Food-borne Vibrio parahemolyticus intoxication A05.4 Food-borne Bacillus cereus -

WO 2009/040088 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 2 April 2009 (02.04.2009) WO 2009/040088 Al (51) International Patent Classification: (74) Agent: ARTH, Hans-Lothar; ABK Patent Attorneys, Jas- A61K 38/08 (2006.01) A61P 25/28 (2006.01) minweg 9, 14052 Berlin (DE). A61P 3/00 (2006.01) A61P 31/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A61P 9/00 (2006.01) A61P 35/00 (2006.01) kind of national protection available): AE, AG, AL, AM, A61P 11/00 (2006.01) A61P 37/00 (2006.01) AO, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY,BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, (21) International Application Number: EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, PCT/EP2008/008007 IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, (22) International Filing Date: MX, MY,MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, PT, 9 September 2008 (09.09.2008) RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY,TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (25) Filing Language: English ZW (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (30) Priority Data: ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, TM), 07017747.2 11 September 2007 (11.09.2007) EP European (AT,BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT,LU, LV,MC, MT, NL, (71) Applicant (for all designated States except US): MONDO- NO, PL, PT, RO, SE, SI, SK, TR), OAPI (BF, BJ, CF, CG, BIOTECH LABORATORIES AG [UfLl]; Herrengasse CI, CM, GA, GN, GQ, GW, ML, MR, NE, SN, TD, TG). -

Experimental Histamine Pruritus
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector EXPERIMENTAL HISTAMINE PRURITUS I. INFLUENCE OF PHYSICAL AND PsYcHoLOGIcAL FACTORS ON THRESHOLD REACTIVTTY* FRANK E. CORMIA, M.D. Itching is by far the most common symptom experienced by patients with skin diseases. It may occur in many clinically unrelated syndromes and is often severe when clinical evi- dence of inflammation is absent. The symptom is particularly common and severe in the various cutaneous manifestations of psychosomatic origin. It is especially important, therefore, that both the qualitative and quantitative aspects of itching be subjected to investigative studies. Pertinent data on the nature of itching has been assembled by Goldscheider (1), Lewis, Grant and Marvin (2), Bickford (3) and others. Much of the literature has been sum- marized by Rothman (4). Recently, Graham, Goodell and Wolff (5) have completed an im- portant re-evaluation of itching. There is now a fairly general agreement that itching is a modified form of pain, carried on afferent pain fibers, with subsequent discharge of impulses up the spinothalamic tracts to the sensory cortex. Cowhage does not produce a pure itch, but a severe itch which contains elements of burning and pricking pain (5). Moreover, the itch produced cannot be quantitatively evaluated. Pruritus produced by histamine ionto- phoresis is equally unreliable. Shelley (6) obtained inconstant results with an electrical stimulator. Rothman, in a personal communication, suggested the use of various dilutions of histamine, administered by the intradermal route. Shelley was able to produce a rather constant degree of itching in a majority of subjects tested by the intradermal injection of a controlled dilution of fresh histamine phosphate solution (7) and made valuable sug- gestions regarding the method (6). -

Serum Iron ICD 10 Codes That Meet Medical Necessity Proprietary Information of Unitedhealthcare Community and State
Serum Iron ICD 10 Codes that Meet Medical Necessity Proprietary Information of UnitedHealthcare Community and State. Copyright 2018 United Healthcare Services, Inc. Unit Codes: CPT Codes: 16027 83540 36009 82728 36134 84446 37642 83550 IRON ICD-10 Codes Covered if selection criteria are met: A01.00 TYPHOID FEVER, UNSPECIFIED A01.01 TYPHOID MENINGITIS A01.02 TYPHOID FEVER WITH HEART INVOLVEMENT A01.03 TYPHOID PNEUMONIA A01.04 TYPHOID ARTHRITIS A01.05 TYPHOID OSTEOMYELITIS A01.09 TYPHOID FEVER WITH OTHER COMPLICATIONS A01.1 PARATYPHOID FEVER A A01.2 PARATYPHOID FEVER B A01.3 PARATYPHOID FEVER C A01.4 PARATYPHOID FEVER, UNSPECIFIED A02.0 SALMONELLA ENTERITIS A02.1 SALMONELLA SEPSIS A02.20 LOCALIZED SALMONELLA INFECTION, UNSPECIFIED A02.21 SALMONELLA MENINGITIS A02.22 SALMONELLA PNEUMONIA A02.23 SALMONELLA ARTHRITIS A02.24 SALMONELLA OSTEOMYELITIS A02.25 SALMONELLA PYELONEPHRITIS A02.29 SALMONELLA WITH OTHER LOCALIZED INFECTION A02.8 OTHER SPECIFIED SALMONELLA INFECTIONS A02.9 SALMONELLA INFECTION, UNSPECIFIED A04.0 ENTEROPATHOGENIC ESCHERICHIA COLI INFECTION A04.1 ENTEROTOXIGENIC ESCHERICHIA COLI INFECTION A04.2 ENTEROINVASIVE ESCHERICHIA COLI INFECTION A04.3 ENTEROHEMORRHAGIC ESCHERICHIA COLI INFECTION A04.4 OTHER INTESTINAL ESCHERICHIA COLI INFECTIONS A04.5 CAMPYLOBACTER ENTERITIS A04.6 ENTERITIS DUE TO YERSINIA ENTEROCOLITICA A04.8 OTHER SPECIFIED BACTERIAL INTESTINAL INFECTIONS A04.9 BACTERIAL INTESTINAL INFECTION, UNSPECIFIED A06.0 ACUTE AMEBIC DYSENTERY A06.1 CHRONIC INTESTINAL AMEBIASIS A06.2 AMEBIC NONDYSENTERIC COLITIS A06.3 AMEBOMA