(12) Patent Application Publication (10) Pub. No.: US 2016/0038462 A1 ZHANG Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
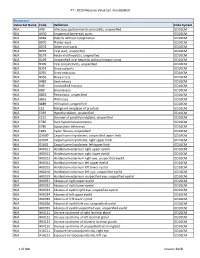
PT - 2020 Measure Value Set Avoidableed
PT - 2020 Measure Value Set_AvoidableED Numerator Value Set Name Code Definition Code System N/A A09 Infectious gastroenteritis and colitis, unspecified ICD10CM N/A A630 Anogenital (venereal) warts ICD10CM N/A B069 Rubella without complication ICD10CM N/A B070 Plantar wart ICD10CM N/A B078 Other viral warts ICD10CM N/A B079 Viral wart, unspecified ICD10CM N/A B179 Acute viral hepatitis, unspecified ICD10CM N/A B199 Unspecified viral hepatitis without hepatic coma ICD10CM N/A B309 Viral conjunctivitis, unspecified ICD10CM N/A B354 Tinea corporis ICD10CM N/A B355 Tinea imbricata ICD10CM N/A B356 Tinea cruris ICD10CM N/A B483 Geotrichosis ICD10CM N/A B49 Unspecified mycosis ICD10CM N/A B80 Enterobiasis ICD10CM N/A B852 Pediculosis, unspecified ICD10CM N/A B853 Phthiriasis ICD10CM N/A B889 Infestation, unspecified ICD10CM N/A C61 Malignant neoplasm of prostate ICD10CM N/A E039 Hypothyroidism, unspecified ICD10CM N/A E215 Disorder of parathyroid gland, unspecified ICD10CM N/A E780 Pure hypercholesterolemia ICD10CM N/A E786 Lipoprotein deficiency ICD10CM N/A E849 Cystic fibrosis, unspecified ICD10CM N/A G5600 Carpal tunnel syndrome, unspecified upper limb ICD10CM N/A G5601 Carpal tunnel syndrome, right upper limb ICD10CM N/A G5602 Carpal tunnel syndrome, left upper limb ICD10CM N/A H00011 Hordeolum externum right upper eyelid ICD10CM N/A H00012 Hordeolum externum right lower eyelid ICD10CM N/A H00013 Hordeolum externum right eye, unspecified eyelid ICD10CM N/A H00014 Hordeolum externum left upper eyelid ICD10CM N/A H00015 Hordeolum externum -

Rwanda ICD-10 Diagnostic Code Subset
Rwanda ICD-10 Diagnostic Code Subset ICD 10 code Diagnosis/Description A Infectious and parasitic diseases A00.0 CHOLERA DUE TO VIBRIO CHOLERAE 01_ BIOVAR CHOLERAE A01 TYPHOID AND PARATYPHOID FEVERS A01.0 TYPHOID FEVER A01.4 PARATYPHOID FEVER_ UNSPECIFIED A02 OTHER SALMONELLA INFECTIONS A02.0 SALMONELLA GASTROENTERITIS A02.1 SALMONELLA SEPTICEMIA A02.2 LOCALIZED SALMONELLA INFECTIONS A02.9 SALMONELLA INFECTION_ UNSPECIFIED A03 SHIGELLOSIS A03.0 SHIGELLOSIS DUE TO SHIGELLA DYSENTERIAE A03.2 SHIGELLOSIS DUE TO SHIGELLA BOYDII A03.9 SHIGELLOSIS_ UNSPECIFIED A04 OTHER BACTERIAL INTESTINAL INFECTIONS A04.0 ENTEROPATHOGENIC ESCHERICHIA COLI INFECTION A04.1 ENTEROTOXIGENIC ESCHERICHIA COLI INFECTION A04.4 OTHER INTESTINAL ESCHERICHIA COLI INFECTIONS A04.5 CAMPYLOBACTER ENTERITIS A04.7 ENTEROCOLITIS DUE TO CLOSTRIDIUM DIFFICILE A04.8 OTHER SPECIFIED BACTERIAL INTESTINAL INFECTIONS A04.9 BACTERIAL INTESTINAL INFECTION_ UNSPECIFIED A05 OTHER BACTERIAL FOOD-BORNE INTOXICATIONS A05.0 FOOD-BORNE STAPHYLOCOCCAL INTOXICATION A05.1 BOTULISM A05.4 FOOD-BORNE BACILLUS CEREUS INTOXICATION A05.8 OTHER SPECIFIED BACTERIAL FOOD-BORNE INTOXICATIONS A05.9 BACTERIAL FOOD-BORNE INTOXICATION_ UNSPECIFIED A06 AMEBIASIS A06.0 ACUTE AMEBIC DYSENTERY A06.1 CHRONIC INTESTINAL AMEBIASIS A06.2 AMEBIC NONDYSENTERIC COLITIS A06.3 AMEBOMA OF INTESTINE A06.4 AMEBIC LIVER ABSCESS A06.5 AMEBIC LUNG ABSCESS A06.6 AMEBIC BRAIN ABSCESS A06.7 CUTANEOUS AMEBIASIS A06.8 AMEBIC INFECTION OF OTHER SITES A06.9 AMEBIASIS_ UNSPECIFIED A07.0 BALANTIDIASIS A07.1 GIARDIASIS [LAMBLIASIS] -

( 12 ) United States Patent
US009974742B2 (12 ) United States Patent (10 ) Patent No. : US 9 , 974 , 742 B2 Ottoboni et al. (45 ) Date of Patent: * May 22, 2018 ( 54 ) EMULSION FORMULATIONS OF AN NK - 1 2013 /0236501 A1 * 9 /2013 Booth . .. .. .. A61K 9 /0019 424 / 400 RECEPTOR ANTAGONIST AND USES 2013 /0317016 AL 11 /2013 Hingorani et al. THEREOF 2016 / 0024092 Al 1 / 2016 Wan et al. 2016 / 0082013 Al 3 / 2016 Ottoboni et al . @(71 ) Applicant : Heron Therapeutics , Inc. , Redwood 2016 /0206622 A1 3 / 2016 Ottoboni et al . City , CA (US ) 2017 / 0112847 AL 4 /2017 Ottoboni et al. @(72 ) Inventors : Thomas B . Ottoboni, Belmont, CA (US ) ; Han Han , Mountain View , CA FOREIGN PATENT DOCUMENTS (US ) CN 102379 * 3 / 2012 CN 102379845 A 3 / 2012 @(73 ) Assignee : Heron Therapeutics, Inc. , San Diego , WO WO 2005 /016308 AL 2 / 2005 WO WO 2009 / 124756 AL 10 / 2009 CA (US ) WO WO 2011 / 158053 AL 12 / 2011 WO WO 2013 / 177501 A2 11 / 2013 @( * ) Notice : Subject to any disclaimer , the term of this WO WO 2014 /0209962 AL 12 /2014 patent is extended or adjusted under 35 WO WO 2014 /005606 AL 3 / 2016 U . S . C . 154 ( b ) by 0 days . days . WO WO 2016 /044784 Al 3 / 2016 This patent is subject to a terminal dis claimer . OTHER PUBLICATIONS (21 ) Appl. No. : 15 /012 , 532 Cassileth et al. in Arch . Intern Med . 1983 ; 143 ( 7 ) : 1347 - 1349 ( Abstract ) . * Dexamethasone Hydrogen Phosphate at web .archive . org/ web / ( 22 ) Filed : Feb . 1 , 2016 20141224130045 /http :/ /www . drugs . com / pro / dexamethasone -so dium -phosphate .html ( retrieved on the internet Mar. -

The Significance of NK1 Receptor Ligands and Their Application In
pharmaceutics Review The Significance of NK1 Receptor Ligands and Their Application in Targeted Radionuclide Tumour Therapy Agnieszka Majkowska-Pilip * , Paweł Krzysztof Halik and Ewa Gniazdowska Centre of Radiochemistry and Nuclear Chemistry, Institute of Nuclear Chemistry and Technology, Dorodna 16, 03-195 Warsaw, Poland * Correspondence: [email protected]; Tel.: +48-22-504-10-11 Received: 7 June 2019; Accepted: 16 August 2019; Published: 1 September 2019 Abstract: To date, our understanding of the Substance P (SP) and neurokinin 1 receptor (NK1R) system shows intricate relations between human physiology and disease occurrence or progression. Within the oncological field, overexpression of NK1R and this SP/NK1R system have been implicated in cancer cell progression and poor overall prognosis. This review focuses on providing an update on the current state of knowledge around the wide spectrum of NK1R ligands and applications of radioligands as radiopharmaceuticals. In this review, data concerning both the chemical and biological aspects of peptide and nonpeptide ligands as agonists or antagonists in classical and nuclear medicine, are presented and discussed. However, the research presented here is primarily focused on NK1R nonpeptide antagonistic ligands and the potential application of SP/NK1R system in targeted radionuclide tumour therapy. Keywords: neurokinin 1 receptor; Substance P; SP analogues; NK1R antagonists; targeted therapy; radioligands; tumour therapy; PET imaging 1. Introduction Neurokinin 1 receptor (NK1R), also known as tachykinin receptor 1 (TACR1), belongs to the tachykinin receptor subfamily of G protein-coupled receptors (GPCRs), also called seven-transmembrane domain receptors (Figure1)[ 1–3]. The human NK1 receptor structure [4] is available in Protein Data Bank (6E59). -

Neurokinin Receptor NK Receptor
Neurokinin Receptor NK receptor There are three main classes of neurokinin receptors: NK1R (the substance P preferring receptor), NK2R, and NK3R. These tachykinin receptors belong to the class I (rhodopsin-like) G-protein coupled receptor (GPCR) family. The various tachykinins have different binding affinities to the neurokinin receptors: NK1R, NK2R, and NK3R. These neurokinin receptors are in the superfamily of transmembrane G-protein coupled receptors (GPCR) and contain seven transmembrane loops. Neurokinin-1 receptor interacts with the Gαq-protein and induces activation of phospholipase C followed by production of inositol triphosphate (IP3) leading to elevation of intracellular calcium as a second messenger. Further, cyclic AMP (cAMP) is stimulated by NK1R coupled to the Gαs-protein. The neurokinin receptors are expressed on many cell types and tissues. www.MedChemExpress.com 1 Neurokinin Receptor Antagonists, Agonists, Inhibitors, Modulators & Activators Aprepitant Befetupitant (MK-0869; MK-869; L-754030) Cat. No.: HY-10052 (Ro67-5930) Cat. No.: HY-19670 Aprepitant (MK-0869) is a selective and Befetupitant is a high-affinity, nonpeptide, high-affinity neurokinin 1 receptor antagonist competitive tachykinin 1 receptor (NK1R) with a Kd of 86 pM. antagonist. Purity: 99.67% Purity: >98% Clinical Data: Launched Clinical Data: No Development Reported Size: 10 mM × 1 mL, 5 mg, 10 mg, 50 mg, 100 mg, 200 mg Size: 1 mg, 5 mg Biotin-Substance P Casopitant mesylate Cat. No.: HY-P2546 (GW679769B) Cat. No.: HY-14405A Biotin-Substance P is the biotin tagged Substance Casopitant mesylate (GW679769B) is a potent, P. Substance P (Neurokinin P) is a neuropeptide, selective, brain permeable and orally active acting as a neurotransmitter and as a neurokinin 1 (NK1) receptor antagonist. -

190.18 - Serum Iron Studies
Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) 190.18 - Serum Iron Studies HCPCS Codes (Alphanumeric, CPT AMA) Code Description 82728 Ferritin 83540 Iron 83550 Iron Binding capacity 84466 Transferrin ICD-10-CM Codes Covered by Medicare Program The ICD-10-CM codes in the table below can be viewed on CMS’ website as part of Downloads: Lab Code List, at http://www.cms.gov/Medicare/Coverage/CoverageGenInfo/LabNCDsICD10.html Code Description A01.00 Typhoid fever, unspecified A01.01 Typhoid meningitis A01.02 Typhoid fever with heart involvement A01.03 Typhoid pneumonia A01.04 Typhoid arthritis A01.05 Typhoid osteomyelitis A01.09 Typhoid fever with other complications A01.1 Paratyphoid fever A A01.2 Paratyphoid fever B A01.3 Paratyphoid fever C A01.4 Paratyphoid fever, unspecified A02.0 Salmonella enteritis A02.1 Salmonella sepsis A02.20 Localized salmonella infection, unspecified NCD 190.18 January 2021 Changes ICD-10-CM Version – Red Fu Associates, Ltd. January 2021 1 Medicare National Coverage Determinations (NCD) Coding Policy Manual and Change Report (ICD-10-CM) Code Description A02.21 Salmonella meningitis A02.22 Salmonella pneumonia A02.23 Salmonella arthritis A02.24 Salmonella osteomyelitis A02.25 Salmonella pyelonephritis A02.29 Salmonella with other localized infection A02.8 Other specified salmonella infections A02.9 Salmonella infection, unspecified A04.0 Enteropathogenic Escherichia coli infection A04.1 Enterotoxigenic Escherichia coli infection A04.2 Enteroinvasive Escherichia -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

The Clinical Conundrum of Pruritus Victoria Garcia-Albea, Karen Limaye
FEATURE ARTICLE The Clinical Conundrum of Pruritus Victoria Garcia-Albea, Karen Limaye ABSTRACT: Pruritus is a common complaint for derma- Pruritus is also the most common symptom in derma- tology patients. Diagnosing the cause of pruritus can tological disease and can be a symptom of several sys- be difficult and is often frustrating for patients and pro- temic diseases (Bernhard, 1994). The overall incidence viders. Even after the diagnosis is made, it can be a chal- of pruritus is unknown because there are no epidemio- lenge to manage and relieve pruritus. This article reviews logical databases for pruritus (Norman, 2003). According common and uncommon causes of pruritus and makes to a 2003 study, pruritus and xerosis are the most com- recommendations for proper and thorough evaluation mon dermatological problems encountered in nursing and management. home patients (Norman, 2003). Key words: Itch, Pruritus INTRODUCTION ETIOLOGY Pruritus (itch) is the most frequent symptom in derma- The skin is equipped with a network of afferent sensory tology (Serling, Leslie, & Maurer, 2011). Itch is the pre- and efferent autonomic nerve branches that respond to dominant symptom associated with acute and chronic various chemical mediators found in the skin (Bernhard, cutaneous disease and is a major symptom in systemic dis- 1994). It is believed that maximal itch production is ease (Elmariah & Lerner, 2011; Steinhoff, Cevikbas, Ikoma, achieved at the basal layer of the epidermis, below which & Berger, 2011). It can be a frustration for both the patient pain is perceived (Bernhard, 1994). Autonomic nerves in- and the clinician. This article will attempt to provide an nervate hair follicles, pili erector muscles, blood vessels, overview of pruritus, a method for thorough and com- eccrine, apocrine, and sebaceous glands (Bernhard, 1994). -

The Neurokinin-1 Receptor Is Expressed with Gastrin-Releasing
Research Articles: Cellular/Molecular The neurokinin-1 receptor is expressed with gastrin-releasing peptide receptor in spinal interneurons and modulates itch https://doi.org/10.1523/JNEUROSCI.1832-20.2020 Cite as: J. Neurosci 2020; 10.1523/JNEUROSCI.1832-20.2020 Received: 15 July 2020 Revised: 25 August 2020 Accepted: 21 September 2020 This Early Release article has been peer-reviewed and accepted, but has not been through the composition and copyediting processes. The final version may differ slightly in style or formatting and will contain links to any extended data. Alerts: Sign up at www.jneurosci.org/alerts to receive customized email alerts when the fully formatted version of this article is published. Copyright © 2020 the authors 1 Title: The neurokinin-1 receptor is expressed with gastrin-releasing peptide receptor in spinal 2 interneurons and modulates itch 3 4 Abbreviated Title: Neurokinin-1 receptor spinal neurons mediate itch 5 6 7 Authors: Tayler D. Sheahan1, Charles A. Warwick1, Louis G. Fanien1, Sarah E. Ross1 8 9 10 1Pittsburgh Center for Pain Research and Department of Neurobiology, University of Pittsburgh, 11 Pennsylvania 12 13 Corresponding author: Sarah E. Ross, Department of Neurobiology, University of Pittsburgh, 14 200 Lothrop Street, Pittsburgh, PA, e-mail: [email protected] 15 The authors have no conflicts of interest to declare. 16 17 18 Number of text pages: 19 19 20 21 Number of figures and tables: 5 figures 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 1 50 Abstract 51 52 The neurokinin-1 receptor (NK1R, encoded by Tacr1) is expressed in spinal dorsal horn 53 neurons and has been suggested to mediate itch in rodents. -

Blood Glucose Testing 2020.Pdf
National Coverage Determination Procedure Code: 82947, 82948, 82962 Blood Glucose Testing CMS Policy Number: 190.20 See also: Medicare Preventive Services Back to NCD List Description: This policy is intended to apply to blood samples used to determine glucose levels. Blood glucose determination may be done using whole blood, serum or plasma. It may be sampled by capillary puncture, as in the fingerstick method, or by vein puncture or arterial sampling. The method for assay may be by color comparison of an indicator stick, by meter assay of whole blood or a filtrate of whole blood, using a device approved for home monitoring, or by using a laboratory assay system using serum or plasma. The convenience of the meter or stick color method allows a patient to have access to blood glucose values in less than a minute or so and has become a standard of care for control of blood glucose, even in the inpatient setting. Indications: Blood glucose values are often necessary for the management of patients with diabetes mellitus, where hyperglycemia and hypoglycemia are often present. They are also critical in the determination of control of blood glucose levels in patient with impaired fasting glucose (FPG 110-125 mg/dL), patient with insulin resistance syndrome and/or carbohydrate intolerance (excessive rise in glucose following ingestion of glucose/glucose sources of food), in patient with a hypoglycemia disorder such as nesidioblastosis or insulinoma, and in patients with a catabolic or malnutrition state. In addition to conditions listed, glucose testing may be medically necessary in patients with tuberculosis, unexplained chronic or recurrent infections, alcoholism, coronary artery disease (especially in women), or unexplained skin conditions (i.e.: pruritis, skin infections, ulceration and gangrene without cause). -

Neurokinin-1 Antagonist Orvepitant for EGFRI- Induced
Open access Original research BMJ Open: first published as 10.1136/bmjopen-2019-030114 on 6 February 2020. Downloaded from Neurokinin-1 antagonist orvepitant for EGFRI- induced pruritus in patients with cancer: a randomised, placebo- controlled phase II trial Bruno Vincenzi ,1 Mike Trower ,2 Ajay Duggal ,3 Pamela Guglielmini ,4 Peter Harris ,2 David Jackson ,5 Mario E Lacouture ,6 Emiliangelo Ratti ,2 Giuseppe Tonini ,1 Andrew Wood ,7 Sonja Ständer 8 To cite: Vincenzi B, Trower M, ABSTRACT Strengths and limitations of this study Duggal A, et al. Neurokinin-1 Objective To evaluate the efficacy of orvepitant (10 or antagonist orvepitant for 30 mg given once daily, orally for 4 weeks), a neurokinin-1 EGFRI- induced pruritus ► The RELIEVE 1 study was the first randomised, in patients with cancer: a receptor antagonist, compared with placebo in reducing double- blind, placebo- controlled study of a neuroki- randomised, placebo- controlled the intensity of epidermal growth factor receptor inhibitor nin-1 antagonist for epidermal growth factor recep- phase II trial. BMJ Open (EGFRI)- induced intense pruritus. tor inhibitor- induced pruritus. 2020;10:e030114. doi:10.1136/ Design Randomised, double- blind, placebo- controlled ► Patients reported scores for the primary endpoint of bmjopen-2019-030114 clinical trial. reduction of itch intensity on a daily basis using an ► Prepublication history for Setting 15 hospitals in Italy and five hospitals in the UK. interactive voice response system. this paper is available online. Participants 44 patients aged ≥18 years receiving an ► Effects on sleep and quality of life were also To view these files, please visit EGFRI for a histologically confirmed malignant solid tumour measured.