WO 2009/040088 Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
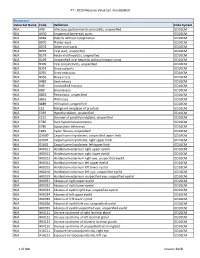
PT - 2020 Measure Value Set Avoidableed
PT - 2020 Measure Value Set_AvoidableED Numerator Value Set Name Code Definition Code System N/A A09 Infectious gastroenteritis and colitis, unspecified ICD10CM N/A A630 Anogenital (venereal) warts ICD10CM N/A B069 Rubella without complication ICD10CM N/A B070 Plantar wart ICD10CM N/A B078 Other viral warts ICD10CM N/A B079 Viral wart, unspecified ICD10CM N/A B179 Acute viral hepatitis, unspecified ICD10CM N/A B199 Unspecified viral hepatitis without hepatic coma ICD10CM N/A B309 Viral conjunctivitis, unspecified ICD10CM N/A B354 Tinea corporis ICD10CM N/A B355 Tinea imbricata ICD10CM N/A B356 Tinea cruris ICD10CM N/A B483 Geotrichosis ICD10CM N/A B49 Unspecified mycosis ICD10CM N/A B80 Enterobiasis ICD10CM N/A B852 Pediculosis, unspecified ICD10CM N/A B853 Phthiriasis ICD10CM N/A B889 Infestation, unspecified ICD10CM N/A C61 Malignant neoplasm of prostate ICD10CM N/A E039 Hypothyroidism, unspecified ICD10CM N/A E215 Disorder of parathyroid gland, unspecified ICD10CM N/A E780 Pure hypercholesterolemia ICD10CM N/A E786 Lipoprotein deficiency ICD10CM N/A E849 Cystic fibrosis, unspecified ICD10CM N/A G5600 Carpal tunnel syndrome, unspecified upper limb ICD10CM N/A G5601 Carpal tunnel syndrome, right upper limb ICD10CM N/A G5602 Carpal tunnel syndrome, left upper limb ICD10CM N/A H00011 Hordeolum externum right upper eyelid ICD10CM N/A H00012 Hordeolum externum right lower eyelid ICD10CM N/A H00013 Hordeolum externum right eye, unspecified eyelid ICD10CM N/A H00014 Hordeolum externum left upper eyelid ICD10CM N/A H00015 Hordeolum externum -

Current Management and Outcome of Tracheobronchial Malacia and Stenosis Presenting to the Paediatric Intensive Care Unit
Intensive Care Med 52001) 27: 722±729 DOI 10.1007/s001340000822 NEONATAL AND PEDIATRIC INTENSIVE CARE David P.Inwald Current management and outcome Derek Roebuck Martin J.Elliott of tracheobronchial malacia and stenosis Quen Mok presenting to the paediatric intensive care unit Abstract Objective: To identify fac- but was not related to any other fac- Received: 10 July 2000 Final Revision received: 14 Oktober 2000 tors associated with mortality and tor. Patients with stenosis required a Accepted: 24 October 2000 prolonged ventilatory requirements significantly longer period of venti- Published online: 16 February 2001 in patients admitted to our paediat- latory support 5median length of Springer-Verlag 2001 ric intensive care unit 5PICU) with ventilation 59 days) than patients tracheobronchial malacia and with malacia 539 days). stenosis diagnosed by dynamic con- Conclusions: Length of ventilation Dr Inwald was supported by the Medical Research Council. This work was jointly trast bronchograms. and bronchographic diagnosis did undertaken in Great Ormond Street Hos- Design: Retrospective review. not predict survival. The only factor pital for Children NHSTrust, which re- Setting: Tertiary paediatric intensive found to contribute significantly to ceived a proportion of its funding from the care unit. mortality was the presence of com- NHSExecutive; the views expressed in this Patients: Forty-eight cases admitted plex cardiac and/or syndromic pa- publication are those of the authors and not to our PICU over a 5-year period in thology. However, patients with necessarily those of the NHSexecutive. whom a diagnosis of tracheobron- stenosis required longer ventilatory chial malacia or stenosis was made support than patients with malacia. -

The Role of Larygotracheal Reconstruction in the Management of Recurrent Croup in Patients with Subglottic Stenosis
International Journal of Pediatric Otorhinolaryngology 82 (2016) 78–80 Contents lists available at ScienceDirect International Journal of Pediatric Otorhinolaryngology jo urnal homepage: www.elsevier.com/locate/ijporl The role of larygotracheal reconstruction in the management of recurrent croup in patients with subglottic stenosis a,b,c, a,b a,d,e Bianca Siegel *, Prasad Thottam , Deepak Mehta a Department of Pediatric Otolaryngology, Childrens Hospital of Pittsburgh of UPMC, Pittsburgh, PA, USA b Children’s Hospital of Michigan, Detroit, MI, USA c Wayne State University School of Medicine Department of Otolaryngology, Detroit, MI, USA d Texas Children’s Hospital, Houston, TX, USA e Baylor University School of Medicine Department of Otolaryngology, Houston, TX, USA A R T I C L E I N F O A B S T R A C T Article history: Objectives: To determine the role of laryngotracheal reconstruction for recurrent croup and evaluate Received 16 October 2015 surgical outcomes in this cohort of patients. Received in revised form 4 January 2016 Methods: Retrospective chart review at a tertiary care pediatric hospital. Accepted 6 January 2016 Results: Six patients who underwent laryngotracheal reconstruction (LTR) for recurrent croup with Available online 13 January 2016 underlying subglottic stenosis were identified through a search of our IRB-approved airway database. At the time of diagnostic bronchoscopy, all 6 patients had grade 2 subglottic stenosis. All patients were Keywords: treated for reflux and underwent esophageal biopsies at the time of diagnostic bronchoscopy; 1 patient Laryngotracheal reconstruction had eosinophilic esophagitis which was treated. All patients had a history of at least 3 episodes of croup Recurrent croup in a 1 year period requiring multiple hospital admissions. -

Diagnostic Issues in Systemic Lupus Erythematosis
266 Postgrad Med J 2001;77:266–285 Postgrad Med J: first published as 10.1136/pmj.77.906.268 on 1 April 2001. Downloaded from SELF ASSESSMENT QUESTIONS Diagnostic issues in systemic lupus erythematosis N Sofat, C Higgens Answers on p 274. A 24 year old woman was diagnosed with sys- (4) What other tests (apart from 24 hour urine temic lupus erythematosis (SLE) based on a creatinine clearance) are available to measure few months’ history of a photosensitive skin the glomerular filtration rate? rash, predominantly on her face, arthralgia The patient had a 24 hour urinary protein col- involving both hands and wrists, a positive lection, which showed a 24 hour protein measure- antinuclear antibody (ANA) test and a raised ment of 1.8 g. There was no evidence of cellular antinative double stranded DNA antibody casts on urine microscopy. Her blood results were binding level. She was treated with oral as below (normal values are in parentheses): hydroxychloroquine 400 mg daily and short x Sodium 134 mmol/l (135–145) courses of prednisolone during flare-ups. x Potassium 4.5 mmol/l (3.5–5.0) She was reviewed in clinic for her regular x Urea 7.0 mmol/l (2.5–6.7) follow up appointment when she was found to x Creatinine 173 µmol/l (70–115) be hypertensive on repeated measurements of x Haemoglobin 108 g/l (115–160) her blood pressure, an average value being x White cell count 4.5 × 109/l (4.0–11.0) 150/90 mm Hg. She was also urine dipstick x Platelets 130 × 109/l (150–400) positive for blood and protein. -

Left Bronchial with Bronchomalacia, Intractable Wheeze
Thorax 1991;46:459-461 459 heart disease.7 This report describes a boy Left bronchial who had had intractable wheezing from infancy as a result of widespread discrete areas isomerism associated of bronchomalacia without bronchiectasis, Thorax: first published as 10.1136/thx.46.6.459 on 1 June 1991. Downloaded from and who also had some minor congenital with bronchomalacia, malformations and a rare combination of bronchial, atrial, and abdominal anatomical presenting with arrangements. We report this case because of the unusual anatomy and other congenital intractable wheeze malformations, and to emphasise the care needed in assessing wheezy children. Philip Lee, Andrew Bush, John 0 Warner Case report This 12 year old boy was referred as a case of steroid resistant asthma. He had had recurrent episodes of coughing and noisy breathing from the age of 5 months, usually precipitated by an upper respiratory infection. At 22 months a Abstract murmur was noted during an episode of right The cause of the Williams Campbell syn- lower lobe pneumonia, and he subsequently drome (bronchomalacia with bronchi- underwent ligation ofa patent arterial duct. He ectasis) is controversial. A boy with subsequently developed wheezing in the early bronchomalacia, bifid ribs, and left bron- morning, a chronic cough, and breathlessness chial isomerism presented with intract- on minimal exertion, despite inhaling sal- able wheeze mimicking asthma. The butamol and beclomethasone. A trial of oral combination of the abdominal, bron- prednisolone, 30 mg daily for one week, failed chial, and atrial anatomy seen in this to improve his symptoms. The only physical child has been described only once finding of note was widespread inspiratory and previously. -

Rwanda ICD-10 Diagnostic Code Subset
Rwanda ICD-10 Diagnostic Code Subset ICD 10 code Diagnosis/Description A Infectious and parasitic diseases A00.0 CHOLERA DUE TO VIBRIO CHOLERAE 01_ BIOVAR CHOLERAE A01 TYPHOID AND PARATYPHOID FEVERS A01.0 TYPHOID FEVER A01.4 PARATYPHOID FEVER_ UNSPECIFIED A02 OTHER SALMONELLA INFECTIONS A02.0 SALMONELLA GASTROENTERITIS A02.1 SALMONELLA SEPTICEMIA A02.2 LOCALIZED SALMONELLA INFECTIONS A02.9 SALMONELLA INFECTION_ UNSPECIFIED A03 SHIGELLOSIS A03.0 SHIGELLOSIS DUE TO SHIGELLA DYSENTERIAE A03.2 SHIGELLOSIS DUE TO SHIGELLA BOYDII A03.9 SHIGELLOSIS_ UNSPECIFIED A04 OTHER BACTERIAL INTESTINAL INFECTIONS A04.0 ENTEROPATHOGENIC ESCHERICHIA COLI INFECTION A04.1 ENTEROTOXIGENIC ESCHERICHIA COLI INFECTION A04.4 OTHER INTESTINAL ESCHERICHIA COLI INFECTIONS A04.5 CAMPYLOBACTER ENTERITIS A04.7 ENTEROCOLITIS DUE TO CLOSTRIDIUM DIFFICILE A04.8 OTHER SPECIFIED BACTERIAL INTESTINAL INFECTIONS A04.9 BACTERIAL INTESTINAL INFECTION_ UNSPECIFIED A05 OTHER BACTERIAL FOOD-BORNE INTOXICATIONS A05.0 FOOD-BORNE STAPHYLOCOCCAL INTOXICATION A05.1 BOTULISM A05.4 FOOD-BORNE BACILLUS CEREUS INTOXICATION A05.8 OTHER SPECIFIED BACTERIAL FOOD-BORNE INTOXICATIONS A05.9 BACTERIAL FOOD-BORNE INTOXICATION_ UNSPECIFIED A06 AMEBIASIS A06.0 ACUTE AMEBIC DYSENTERY A06.1 CHRONIC INTESTINAL AMEBIASIS A06.2 AMEBIC NONDYSENTERIC COLITIS A06.3 AMEBOMA OF INTESTINE A06.4 AMEBIC LIVER ABSCESS A06.5 AMEBIC LUNG ABSCESS A06.6 AMEBIC BRAIN ABSCESS A06.7 CUTANEOUS AMEBIASIS A06.8 AMEBIC INFECTION OF OTHER SITES A06.9 AMEBIASIS_ UNSPECIFIED A07.0 BALANTIDIASIS A07.1 GIARDIASIS [LAMBLIASIS] -

Revision Tracheobronchoplasty: Case Report
4 Case Report Page 1 of 4 Revision tracheobronchoplasty: case report Ammara A. Watkins, Jennifer L. Wilson, Mihir Parikh, Adnan Majid, Sidhu P. Gangadharan Division of Thoracic Surgery and Interventional Pulmonology, Beth Israel Deaconess, Harvard Medical School, Boston, MA, USA Correspondence to: Sidhu P. Gangadharan, MD. Chief, Division of Thoracic Surgery and Interventional Pulmonology, Beth Israel Deaconess Medical Center, 185 Pilgrim Rd. W/DC 201 Boston, MA 02215, USA. Email: [email protected]. Abstract: Tracheobronchoplasty, or posterior splinting of the airway with mesh, is a durable solution for patients with severe tracheobronchomalacia (TBM). Recurrent symptoms of TBM following tracheobronchoplasty are uncommon; however, when they occur can have significant impact on quality of life. Appropriate management of recurrent TBM requires a systematic and multidisciplinary collaborative approach. We present a patient with postoperative symptom recurrence requiring revisional tracheobronchoplasty to highlight the complexity of the disease’s presentation, workup and treatment. Keywords: Reoperative; revision; tracheobronchoplasty; tracheobronchomalacia (TBM); case report Received: 06 October 2019; Accepted: 18 December 2019; Published: 25 November 2020. doi: 10.21037/ccts.2019.12.14 View this article at: http://dx.doi.org/10.21037/ccts.2019.12.14 Introduction her tracheobronchoplasty she reported recurrent wheezing, cough and shortness of breath. By four years following Tracheobronchomalacia is an increasingly recognized her operation, the progressive symptoms considerably abnormality of the central airway that can cause dyspnea, impacted her quality of life. She was unable to walk 2 cough, recurrent respiratory infections and respiratory blocks without shortness of breath and had been admitted insufficiency (1,2). The hallmark of the disease is expiratory at least six times in the past year due to respiratory distress. -
![[Intrinsic] Tracheomalacia in Children](https://docslib.b-cdn.net/cover/1748/intrinsic-tracheomalacia-in-children-681748.webp)
[Intrinsic] Tracheomalacia in Children
Interventions for primary (intrinsic) tracheomalacia in children (Review) Masters IB, Chang AB This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2005, Issue 4 http://www.thecochranelibrary.com Interventions for primary (intrinsic) tracheomalacia in children (Review) Copyright © 2008 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 3 METHODS ...................................... 3 RESULTS....................................... 5 DISCUSSION ..................................... 5 AUTHORS’CONCLUSIONS . 6 ACKNOWLEDGEMENTS . 6 REFERENCES ..................................... 6 CHARACTERISTICSOFSTUDIES . 7 DATAANDANALYSES. 9 ADDITIONALTABLES. 9 WHAT’SNEW..................................... 9 HISTORY....................................... 10 CONTRIBUTIONSOFAUTHORS . 10 DECLARATIONSOFINTEREST . 10 SOURCESOFSUPPORT . 10 INDEXTERMS .................................... 10 Interventions for primary (intrinsic) tracheomalacia in children (Review) i Copyright © 2008 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. [Intervention Review] Interventions for primary (intrinsic) tracheomalacia in children I Brent Masters1, Anne B Chang2 1Respiratory Medicine, Royal Children’s Hospital, Brisbane, Australia. -

ERS Statement on Tracheomalacia and Bronchomalacia in Children
ERS OFFICIAL DOCUMENT ERS STATEMENT ERS statement on tracheomalacia and bronchomalacia in children Colin Wallis1,EfthymiaAlexopoulou2,JuanL.Antón-Pacheco3,JayeshM.Bhatt 4, Andrew Bush5,AnneB.Chang6,7,8,Anne-MarieCharatsi9, Courtney Coleman10, Julie Depiazzi11, Konstantinos Douros12,ErnstEber13,MarkEverard14, Ahmed Kantar15,IanB.Masters6,7,FabioMidulla16, Raffaella Nenna 16,17, Derek Roebuck18, Deborah Snijders19 and Kostas Priftis12 @ERSpublications This statement provides a comprehensive review of the causes, presentation, recognition and management of children with tracheobronchomalacia written by a multidisciplinary Task Force in keeping with ERS methodology http://bit.ly/2LPTQCk Cite this article as: Wallis C, Alexopoulou E, Antón-Pacheco JL, et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur Respir J 2019; 54: 1900382 [https://doi.org/10.1183/13993003.00382- 2019]. ABSTRACT Tracheomalacia and tracheobronchomalacia may be primary abnormalities of the large airways or associated with a wide variety of congenital and acquired conditions. The evidence on diagnosis, classification and management is scant. There is no universally accepted classification of severity. Clinical presentation includes early-onset stridor or fixed wheeze, recurrent infections, brassy cough and even near-death attacks, depending on the site and severity of the lesion. Diagnosis is usually made by flexible bronchoscopy in a free-breathing child but may also be shown by other dynamic imaging techniques such as low-contrast volume bronchography, computed tomography or magnetic resonance imaging. Lung function testing can provide supportive evidence but is not diagnostic. Management may be medical or surgical, depending on the nature and severity of the lesions, but the evidence base for any therapy is limited. While medical options that include bronchodilators, anti-muscarinic agents, mucolytics and antibiotics (as well as treatment of comorbidities and associated conditions) are used, there is currently little evidence for benefit. -

Respiratory Complications and Goldenhar Syndrome
breathe case presentations.qxd 06/03/2007 17:56 Page 15 CASE PRESENTATION Respiratory complications and Goldenhar syndrome Case report W. Jacobs1 A 29-year-old female was referred to hospital A. Vonk Noordegraaf1 with progressive asthmatic complaints. On pre- R.P. Golding2 sentation, the patient had been experiencing J.G. van den Aardweg3 orthopnoea, an audible wheeze during daily P.E. Postmus1 activities, and sporadic coughing without spu- tum production. The patient had a history of recurrent airway infections and a 5-kg weight Depts of 1Pulmonary Medicine loss during the previous year, and had stopped and 2Radiology, Vrije Universiteit smoking several years before. She was known to Medisch Centrum, Amsterdam, have oculo-auriculo-vertebral (OAV) syndrome, and 3Dept of Pulmonary i.e. Goldenhar syndrome, which is a develop- Medicine, Medisch Centrum mental disorder involving mainly first and sec- Alkmaar, The Netherlands. ond branchial arch anomalies. On physical examination, she was not dys- pnoeic at rest, and had a respiratory rate of Correspondence: -1 -1 14 breaths·min , pulse 80 beats·min , blood W. Jacobs pressure 110/70 mmHg and temperature Dept of Pulmonary Medicine Figure 1 37.9°C. The left hemifacial structures and the Vrije Universiteit Medisch Centrum Postero-anterior chest radiograph. left hemithorax were underdeveloped. A chest Postbus 7057 examination revealed a systolic heart murmur 1007 MB Amsterdam grade 2/6 over the apex, and inspiratory and The Netherlands expiratory wheezing over both lungs. There was Task 1 Fax: 31 204444328 E-mail: [email protected] no oedema or clubbing. Arterial blood gas analy- Interpret the chest radiograph. -

Tracheobronchomalacia and Excessive Dynamic Airway Collapse
Blackwell Publishing AsiaMelbourne, AustraliaRESRespirology1323-77992006 Blackwell Publishing Asia Pty Ltd? 2006114388406Review ArticleTBM and EDACSD Murgu and HG Colt Respirology (2006) 11, 388–406 doi: 10.1111/j.1400-1843.2006.00862.x REVIEW ARTICLE Tracheobronchomalacia and excessive dynamic airway collapse Septimiu D. MURGU AND Henri G. COLT Pulmonary and Critical Care Medicine, Department of Medicine, University of California School of Medicine, Irvine, CA, USA Tracheobronchomalacia and excessive dynamic airway collapse MURGU SD, COLT HG. Respirology 2006; 11: 388–406 Abstract: Tracheobronchomalacia and excessive dynamic airway collapse are two separate forms of dynamic central airway obstruction that may or may not coexist. These entities are increasingly recognized as asthma and COPD imitators. The understanding of these disease processes, however, has been compromised over the years because of uncertainties regarding their definitions, patho- genesis and aetiology. To date, there is no standardized classification, diagnosis or management algorithm. In this article we comprehensively review the aetiology, morphopathology, physiology, diagnosis and treatment of these entities. Key words: airflow dynamics, bronchomalacia, excessive dynamic airway collapse, tracheobron- chomalacia, tracheomalacia. INTRODUCTION first is that most published studies are case series and retrospective descriptions, many of which report a The purpose of this systematic review is to clarify con- single investigator’s experience with diagnosis and founding issues pertaining to the definition, patho- management. In fact, it is puzzling that despite the physiology, histopathology, aetiology, diagnosis, relative frequency with which TBM and EDAC are pre- classification and treatment of acquired and idio- sumably encountered, multi-institutional or prospec- pathic forms of adult tracheobronchomalacia (TBM) tive studies have not been published. -

Series of Laryngomalacia, Tracheomalacia, and Bronchomalacia Disorders and Their Associations with Other Conditions in Children
Pediatric Pulmonology 34:189-195 (2002) Series of Laryngomalacia, Tracheomalacia, and Bronchomalacia Disorders and Their Associations With Other Conditions in Children I.B. Masters, MBBS, FRACP,1* A.B. Chang, PhD, FRACP,2 L. Patterson, MBBS, FANZCAC,1 С Wainwright, MD, FRACP,1 H. Buntain, MBBS,1 B.W. Dean, MSC,1 and P.W. Francis, MD, FRACP1 Summary. Laryngomalacia, bronchomalacia, and tracheomalacia are commonly seen in pediatric respiratory medicine, yet their patterns and associations with other conditions are not well-understood. We prospectively video-recorded bronchoscopic data and clinical information from referred patients over a 10-year period and defined aspects of interrelationships and associations. Two hundred and ninety-nine cases of malacia disorders (34%) were observed in 885 bronchoscopic procedures. Cough, wheeze, stridor, and radiological changes were the most common symptoms and signs. The lesions were most often found in males (2:1) and on the left side (1.6:1). Concomitant malacia lesions ranged from 24%forlaryngotracheobronchomalaciato 47% for tracheobronchomalacia. The lesions were found in association with other disorders such as congenital heart disorders (13.7%), tracheo-esophageal fistula (9.6%), and various syndromes (8%). Even though the understanding of these disorders is in its infancy, pediatricians should maintain a level of awareness for malacia lesions and consider the possibility of multiple lesions being present, even when one symptom predominates or occurs alone. Pediatr Pulmonol Pediatr Pulmonol. 2002; 34:189-195. © 2002 wiiey-Liss. inc. Key words: laryngomalacia; tracheomalacia; bronchomalacia; malacia disorders; syndromes. INTRODUCTION The aim of this report is to describe an extensive experience of various forms of laryngomalacia, tracheo Tracheomalacia, bronchomalacia, and laryngomalacia malacia, and bronchomalacia and explore some of the disorders are commonly seen in tertiary pediatric respira interrelationships that exist between these conditions with tory practice.