Delayed Fungal Evolution Did Not Cause the Paleozoic Peak in Coal Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Origin and Early Evolution of Plants on Land
review article The origin and early evolution of plants on land Paul Kenrick & Peter R. Crane . The origin and early evolution of land plants in the mid-Palaeozoic era, between about 480 and 360 million years ago, was an important event in the history of life, with far-reaching consequences for the evolution of terrestrial organisms and global environments. A recent surge of interest, catalysed by palaeobotanical discoveries and advances in the systematics of living plants, provides a revised perspective on the evolution of early land plants and suggests new directions for future research. The origin and early diversification of land plants marks an interval Eoembryophytic (mid-Ordovician [early Llanvirn: ϳ476 Myr] to of unparalleled innovation in the history of plant life. From a simple Early Silurian [late Llandovery: ϳ432 Myr])3. Spore tetrads (com- plant body consisting of only a few cells, land plants (liverworts, prising four membrane-bound spores; Fig. 2d) appear over a broad hornworts, mosses and vascular plants) evolved an elaborate two- geographic area in the mid-Ordovician and provide the first good phase life cycle and an extraordinary array of complex organs and evidence of land plants3,26,29. The combination of a decay-resistant tissue systems. Specialized sexual organs (gametangia), stems with wall (implying the presence of sporopollenin) and tetrahedral an intricate fluid transport mechanism (vascular tissue), structural configuration (implying haploid meiotic products) is diagnostic tissues (such as wood), epidermal structures for respiratory gas of land plants. The precise relationships of the spore producers exchange (stomates), leaves and roots of various kinds, diverse within land plants are controversial, but evidence of tetrads and spore-bearing organs (sporangia), seeds and the tree habit had all other spore types (such as dyads) in Late Silurian and Devonian evolved by the end of the Devonian period. -

International Organisation of Palaeobotany IOP NEWSLETTER
INTERNATIONAL UNION OF BIOLOGIC A L S C IENC ES S ECTION FOR P A L A EOBOTANY International Organisation of Palaeobotany IOP NEWSLETTER 110 August 2016 CONTENTS FROM THE SECRETARY/TREASURER IPC XIV/IOPC X 2016 IOPC 2020 IOP MEMBERSHIP IOP EXECUTIVE COMMITTEE ELECTIONS IOP WEBMASTER POSITION WHAT HAPPENED TO THE OUPH COLLECTIONS? THE PALAEOBOTANY OF ITALY UPCOMING MEETINGS CALL FOR NEWS and NOTES The views expressed in the newsletter are those of its correspondents, and do not necessarily reflect the policy of IOP. Please send us your contributions for the next edition of our newsletter (June 2016) by M ay 30th, 2016. President: Johanna Eder-Kovar (G ermany) Vice Presidents: Bob Spicer (Great Britain), Harufumi Nishida (Japan), M ihai Popa (Romania) M embers at Large: Jun W ang (China), Hans Kerp (Germany), Alexej Herman (Russia) Secretary/Treasurer/Newsletter editor: M ike Dunn (USA) Conference/Congress Chair: Francisco de Assis Ribeiro dos Santos IOP Logo: The evolution of plant architecture (© by A. R. Hemsley) I OP 110 2 August 2016 FROM THE In addition, please send any issues that you think need to be addressed at the Business SECRETARY/TREASURER meeting. I will add those to the Agenda. Dear IOP Members, Respectfully, Mike I am happy to report, that IOP seems to be on track and ready for a new Executive Council to take over. The elections are IPC XIV/IOPC X 2016 progressing nicely and I will report the results in the September/October Newsletter. The one area that is still problematic is the webmaster position. We really to talk amongst ourselves, and find someone who is willing and able to do the job. -

The Carboniferous Evolution of Nova Scotia
Downloaded from http://sp.lyellcollection.org/ by guest on September 27, 2021 The Carboniferous evolution of Nova Scotia J. H. CALDER Nova Scotia Department of Natural Resources, PO Box 698, Halifax, Nova Scotia, Canada B3J 2T9 Abstract: Nova Scotia during the Carboniferous lay at the heart of palaeoequatorial Euramerica in a broadly intermontane palaeoequatorial setting, the Maritimes-West-European province; to the west rose the orographic barrier imposed by the Appalachian Mountains, and to the south and east the Mauritanide-Hercynide belt. The geological affinity of Nova Scotia to Europe, reflected in elements of the Carboniferous flora and fauna, was mirrored in the evolution of geological thought even before the epochal visits of Sir Charles Lyell. The Maritimes Basin of eastern Canada, born of the Acadian-Caledonian orogeny that witnessed the suture of Iapetus in the Devonian, and shaped thereafter by the inexorable closing of Gondwana and Laurasia, comprises a near complete stratal sequence as great as 12 km thick which spans the Middle Devonian to the Lower Permian. Across the southern Maritimes Basin, in northern Nova Scotia, deep depocentres developed en echelon adjacent to a transform platelet boundary between terranes of Avalon and Gondwanan affinity. The subsequent history of the basins can be summarized as distension and rifting attended by bimodal volcanism waning through the Dinantian, with marked transpression in the Namurian and subsequent persistence of transcurrent movement linking Variscan deformation with Mauritainide-Appalachian convergence and Alleghenian thrusting. This Mid- Carboniferous event is pivotal in the Carboniferous evolution of Nova Scotia. Rapid subsidence adjacent to transcurrent faults in the early Westphalian was succeeded by thermal sag in the later Westphalian and ultimately by basin inversion and unroofing after the early Permian as equatorial Pangaea finally assembled and subsequently rifted again in the Triassic. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -

Transformative Paleobotany
Chapter 6 Lower Permian Flora of the Sanzenbacher Ranch, Clay County, Texas William A. DiMichele1, Robert W. Hook2, Hans Kerp3, Carol L. Hotton1,4, Cindy V. Looy5 and Dan S. Chaney1 1NMNH Smithsonian Institution, Washington, DC, United States; 2The University of Texas at Austin, Austin, TX, United States; 3Westfälische Wilhelms-Universität Münster, Münster, Germany; 4National Institutes of Health, Bethesda, MD, United States; 5University of California Berkeley, Berkeley, CA, United States 1. INTRODUCTION 1985; Broutin, 1986; Popa, 1999; Steyer et al., 2000; Wagner and Mayoral, 2007; Bercovici and Broutin, 2008; Since 1989, field parties supported by the U.S. National Barthel, 2009; Wagner and Álvarez-Vázquez, 2010; Museum of Natural History have obtained large collections Barthel and Brauner, 2015). Furthermore, because this of mainly Permian plant fossils from north central Texas. locality was collected on three occasions over a time period This work was undertaken to study known localities and to of 50 years and by different parties, comparative analysis of find new fossiliferous deposits that would contribute to a the Sanzenbacher collections provides a basis for assessing better understanding of floral and paleoenvironmental sites that have comparable histories. changes within the region during the early Permian. From the outset, the effort was interdisciplinary and grew, through the contributions of nearly 20 paleobotanists, 2. GEOLOGY palynologists, invertebrate and vertebrate paleontologists, Clay County is the only county in the Permo-Carboniferous and sedimentary geologists of several subdisciplines, to be outcrop belt of north central Texas that lacks marine rocks. quite comprehensive. Our reporting of results, however, has These alluvial sediments accumulated east of a broad been influenced by unexpected developments, including the coastal plain that bordered the Eastern Shelf of the Midland discovery of new plant-fossil assemblages in areas once Basin. -
M23 Tree History Workbook Pub 2018
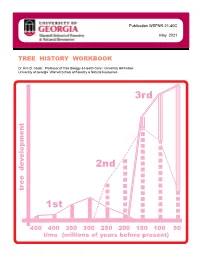
Publication WSFNR-21-40C May 2021 TREE HISTORY WORKBOOK Dr. Kim D. Coder, Professor of Tree Biology & Health Care / University Hill Fellow University of Georgia Warnell School of Forestry & Natural Resources 3rd 2nd tree development 1st 450 400 350 300 250 200 150 100 50 time (millions of years before present) This workbook is an educational product designed for helping people interested in trees to appreciate and understand how modern trees developed across long time periods. This product is a synthesis and integration of research and educational concepts regarding tree evolution and species inception. This educational workbook was designed for awareness building and foundation training of professional tree health care providers. At the time it was finished, this workbook contained educational materials and models concerning tree development thought by the author to provide the best means for considering the most basic understandings of time impacts on tree species creation, extinction, and tree biological and mechanical fundamentals. The University of Georgia, the Warnell School of Forestry & Natural Resources, and the author are not responsible for any errors, omissions, misinterpretations, or misapplications stemming from this educational product. The author assumed all users would have some basic tree biological, structural, and ecological background. This product was not designed, nor is suited, as a literature or research review. Please use the selected literature section at the back of this workbook to find scientific sources. This publication is copyrighted by the author. This educational product is only for noncommercial, nonprofit use and may not be copied or reproduced in whole or in part, by any means, in any format, or in any media including electronic forms, without explicit written permission of the author. -

Diversity and Evolution of the Megaphyll in Euphyllophytes
G Model PALEVO-665; No. of Pages 16 ARTICLE IN PRESS C. R. Palevol xxx (2012) xxx–xxx Contents lists available at SciVerse ScienceDirect Comptes Rendus Palevol w ww.sciencedirect.com General palaeontology, systematics and evolution (Palaeobotany) Diversity and evolution of the megaphyll in Euphyllophytes: Phylogenetic hypotheses and the problem of foliar organ definition Diversité et évolution de la mégaphylle chez les Euphyllophytes : hypothèses phylogénétiques et le problème de la définition de l’organe foliaire ∗ Adèle Corvez , Véronique Barriel , Jean-Yves Dubuisson UMR 7207 CNRS-MNHN-UPMC, centre de recherches en paléobiodiversité et paléoenvironnements, 57, rue Cuvier, CP 48, 75005 Paris, France a r t i c l e i n f o a b s t r a c t Article history: Recent paleobotanical studies suggest that megaphylls evolved several times in land plant st Received 1 February 2012 evolution, implying that behind the single word “megaphyll” are hidden very differ- Accepted after revision 23 May 2012 ent notions and concepts. We therefore review current knowledge about diverse foliar Available online xxx organs and related characters observed in fossil and living plants, using one phylogenetic hypothesis to infer their origins and evolution. Four foliar organs and one lateral axis are Presented by Philippe Taquet described in detail and differ by the different combination of four main characters: lateral organ symmetry, abdaxity, planation and webbing. Phylogenetic analyses show that the Keywords: “true” megaphyll appeared at least twice in Euphyllophytes, and that the history of the Euphyllophytes Megaphyll four main characters is different in each case. The current definition of the megaphyll is questioned; we propose a clear and accurate terminology in order to remove ambiguities Bilateral symmetry Abdaxity of the current vocabulary. -

Megaphylls, Microphylls and the Evolution of Leaf Development
Opinion Megaphylls, microphylls and the evolution of leaf development Alexandru M.F. Tomescu Department of Biological Sciences, Humboldt State University, Arcata, CA 95521, USA Originally coined to emphasize morphological differ- However, the microphyll–megaphyll divide is not as ences, ‘microphyll’ and ‘megaphyll’ became synon- clear cut, and morphological definitions that contrast ymous with the idea that vascular plant leaves are not microphylls and megaphylls as mutually exclusive con- homologous. Although it is now accepted that leaves cepts of leaves are inconsistent. Moreover, current under- evolved independently in several euphyllophyte standing of plant phylogeny and leaf development fails to lineages, ‘megaphyll’ has grown to reflect another type shed light on the origin of microphylls, and supports of homology, that of euphyllophyte leaf precursor struc- several independent origins of megaphylls. Here, I review tures. However, evidence from the fossil record and plant phylogeny and developmental data from fossil and developmental pathways fails to indicate homology extant trachephytes, as well as the current understanding and suggests homoplasy of precursor structures. Thus, of genetic pathways controlling leaf development, to argue as I discuss here, ‘megaphyll’ should be abandoned that the megaphyll concept should be abandoned because because it perpetuates an unsupported idea of it perpetuates misconceptions and confusion based on homology, leading to misconceptions that pervade plant unsupported homology. biology thinking and can bias hypothesis and inference in developmental and phylogenetic studies. Alternative Morphological inconsistencies and overlap in the definitions are needed that are based on development microphyll–megaphyll dichotomy and phylogeny for different independently evolved leaf Microphylls are defined as leaves of small size, with simple types. -

Palynology, Microfacies and Ostracods of the Permian–Triassic Boundary Interval in the Rosengarten/Catinaccio Massif (Southern Alps, Italy)
Austrian Journal of Earth Sciences Vienna 2019 Volume 112/2 103 - 124 DOI: 10.17738/ajes.2019.0007 Palynology, microfacies and ostracods of the Permian–Triassic boundary interval in the Rosengarten/Catinaccio Massif (Southern Alps, Italy) Hendrik NOWAK1)*, Wolfgang METTE2), Fabio M. PETTI3), Guido ROGHI4), Evelyn KUSTATSCHER1),5),6) 1) Museum of Nature South Tyrol, Bindergasse/Via Bottai 1, 39100 Bozen/Bolzano, Italy; e-mail: [email protected]; evelyn.kustatscher@ naturmuseum.it 2) Department of Geology, Universität Innsbruck, Innrain 52f, 6020 Innsbruck, Austria; e-mail: [email protected] 3) MUSE – Museo delle Scienze di Trento, Corso del Lavoro e della Scienza 3, Trento 38122, Italy; e-mail: [email protected] 4) Istituto di Geoscienze e Georisorse - CNR, Via Gradenigo 6, Padova 35131, Italy; e-mail: [email protected] 5) Department of Earth and Environmental Sciences, Paleontology & Geobiology, Ludwig-Maximilians-Universität München, Richard-Wagner-Straße 10, 80333 München, Germany 6) SNSB-Bayerische Staatssammlung für Paläontologie und Geologie, Richard-Wagner-Straße 10, 80333 München, Germany *) Corresponding author: Hendrik NOWAK KEYWORDS upper Permian, Lopingian, Lower Triassic, Dolomites, Bellerophon Formation, Werfen Formation. Abstract The Laurinswand section in the Rosengarten/Catinaccio Massif (Dolomites, Southern Alps, Italy) covers the Permian– Triassic boundary in a proximal marine setting. The section has been studied for palynology, ostracods and carbonate microfacies. Five microfacies types are defined for the carbonates of the Bellerophon Formation (Changhsingian) in this section. Ostracod assemblages from the upper Bellerophon Formation show a moderate to high diversity and mostly indi- cate normal marine conditions, with some samples from the upper Casera Razzo Member being dominated by eurytopic forms. -

Annual Review of Pteridological Research - 2005
Annual Review of Pteridological Research - 2005 Annual Review of Pteridological Research - 2005 Literature Citations All Citations 1. Acosta, S., M. L. Arreguín, L. D. Quiroz & R. Fernández. 2005. Ecological and floristic analysis of the Pteridoflora of the Valley of Mexico. P. 597. In Abstracts (www.ibc2005.ac.at). XVII International Botanical Congress 17–23 July, Vienna Austria. [Abstract] 2. Agoramoorthy, G. & M. J. Hsu. 2005. Borneo's proboscis monkey – a study of its diet of mineral and phytochemical concentrations. Current Science (Bangalore) 89: 454–457. [Acrostichum aureum] 3. Aguraiuja, R. 2005. Hawaiian endemic fern lineage Diellia (Aspleniaceae): distribution, population structure and ecology. P. 111. In Dissertationes Biologicae Universitatis Tartuensis 112. Tartu University Press, Tartu Estonia. 4. Al Agely, A., L. Q. Ma & D. M. Silvia. 2005. Mycorrhizae increase arsenic uptake by the hyperaccumulator Chinese brake fern (Pteris vittata L.). Journal of Environmental Quality 34: 2181–2186. 5. Albertoni, E. F., C. Palma–Silva & C. C. Veiga. 2005. Structure of the community of macroinvertebrates associated with the aquatic macrophytes Nymphoides indica and Azolla filiculoides in two subtropical lakes (Rio Grande, RS, Brazil). Acta Biologica Leopoldensia 27: 137–145. [Portuguese] 6. Albornoz, P. L. & M. A. Hernandez. 2005. Anatomy and mycorrhiza in Pellaea ternifolia (Cav.) Link (Pteridaceae). Bol. Soc. Argent. Bot. 40 (Supl.): 193. [Abstract; Spanish] 7. Almendros, G., M. C. Zancada, F. J. Gonzalez–Vila, M. A. Lesiak & C. Alvarez–Ramis. 2005. Molecular features of fossil organic matter in remains of the Lower Cretaceous fern Weichselia reticulata from Przenosza basement (Poland). Organic Geochemistry 36: 1108–1115. 8. Alonso–Amelot, M. E. -

Anatomy, Affinities, and Evolutionary Implications of New Silicified Stems
geodiversitas 2019 ● 41 ● 14 DIRECTEUR DE LA PUBLICATION : Bruno David, Président du Muséum national d’Histoire naturelle RÉDACTEUR EN CHEF / EDITOR-IN-CHIEF : Didier Merle ASSISTANTS DE RÉDACTION / ASSISTANT EDITORS : Emmanuel Côtez ([email protected]) ; Anne Mabille MISE EN PAGE / PAGE LAYOUT : Emmanuel Côtez COMITÉ SCIENTIFIQUE / SCIENTIFIC BOARD : Christine Argot (MNHN, Paris) Beatrix Azanza (Museo Nacional de Ciencias Naturales, Madrid) Raymond L. Bernor (Howard University, Washington DC) Alain Blieck (chercheur CNRS retraité, Haubourdin) Henning Blom (Uppsala University) Jean Broutin (UPMC, Paris) Gaël Clément (MNHN, Paris) Ted Daeschler (Academy of Natural Sciences, Philadelphie) Bruno David (MNHN, Paris) Gregory D. Edgecombe (The Natural History Museum, Londres) Ursula Göhlich (Natural History Museum Vienna) Jin Meng (American Museum of Natural History, New York) Brigitte Meyer-Berthaud (CIRAD, Montpellier) Zhu Min (Chinese Academy of Sciences, Pékin) Isabelle Rouget (UPMC, Paris) Sevket Sen (MNHN, Paris) Stanislav Štamberg (Museum of Eastern Bohemia, Hradec Králové) Paul Taylor (The Natural History Museum, Londres) COUVERTURE / COVER : Made from the figures of the article. Geodiversitas est indexé dans / Geodiversitas is indexed in: – Science Citation Index Expanded (SciSearch®) – ISI Alerting Services® – Current Contents® / Physical, Chemical, and Earth Sciences® – Scopus® Geodiversitas est distribué en version électronique par / Geodiversitas is distributed electronically by: – BioOne® (http://www.bioone.org) Les articles ainsi