Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Origin and Early Evolution of Plants on Land
review article The origin and early evolution of plants on land Paul Kenrick & Peter R. Crane . The origin and early evolution of land plants in the mid-Palaeozoic era, between about 480 and 360 million years ago, was an important event in the history of life, with far-reaching consequences for the evolution of terrestrial organisms and global environments. A recent surge of interest, catalysed by palaeobotanical discoveries and advances in the systematics of living plants, provides a revised perspective on the evolution of early land plants and suggests new directions for future research. The origin and early diversification of land plants marks an interval Eoembryophytic (mid-Ordovician [early Llanvirn: ϳ476 Myr] to of unparalleled innovation in the history of plant life. From a simple Early Silurian [late Llandovery: ϳ432 Myr])3. Spore tetrads (com- plant body consisting of only a few cells, land plants (liverworts, prising four membrane-bound spores; Fig. 2d) appear over a broad hornworts, mosses and vascular plants) evolved an elaborate two- geographic area in the mid-Ordovician and provide the first good phase life cycle and an extraordinary array of complex organs and evidence of land plants3,26,29. The combination of a decay-resistant tissue systems. Specialized sexual organs (gametangia), stems with wall (implying the presence of sporopollenin) and tetrahedral an intricate fluid transport mechanism (vascular tissue), structural configuration (implying haploid meiotic products) is diagnostic tissues (such as wood), epidermal structures for respiratory gas of land plants. The precise relationships of the spore producers exchange (stomates), leaves and roots of various kinds, diverse within land plants are controversial, but evidence of tetrads and spore-bearing organs (sporangia), seeds and the tree habit had all other spore types (such as dyads) in Late Silurian and Devonian evolved by the end of the Devonian period. -

Fossils and Plant Phylogeny1
American Journal of Botany 91(10): 1683±1699. 2004. FOSSILS AND PLANT PHYLOGENY1 PETER R. CRANE,2,5 PATRICK HERENDEEN,3 AND ELSE MARIE FRIIS4 2Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK; 3Department of Biological Sciences, The George Washington University, Washington DC 20052 USA; 4Department of Palaeobotany, Swedish Museum of Natural History, Box 50007, S-104 05 Stockholm, Sweden Developing a detailed estimate of plant phylogeny is the key ®rst step toward a more sophisticated and particularized understanding of plant evolution. At many levels in the hierarchy of plant life, it will be impossible to develop an adequate understanding of plant phylogeny without taking into account the additional diversity provided by fossil plants. This is especially the case for relatively deep divergences among extant lineages that have a long evolutionary history and in which much of the relevant diversity has been lost by extinction. In such circumstances, attempts to integrate data and interpretations from extant and fossil plants stand the best chance of success. For this to be possible, what will be required is meticulous and thorough descriptions of fossil material, thoughtful and rigorous analysis of characters, and careful comparison of extant and fossil taxa, as a basis for determining their systematic relationships. Key words: angiosperms; fossils; paleobotany; phylogeny; spermatophytes; tracheophytes. Most biological processes, such as reproduction or growth distic context, neither fossils nor their stratigraphic position and development, can only be studied directly or manipulated have any special role in inferring phylogeny, and although experimentally using living organisms. Nevertheless, much of more complex models have been developed (see Fisher, 1994; what we have inferred about the large-scale processes of plant Huelsenbeck, 1994), these have not been widely adopted. -

International Organisation of Palaeobotany IOP NEWSLETTER
INTERNATIONAL UNION OF BIOLOGIC A L S C IENC ES S ECTION FOR P A L A EOBOTANY International Organisation of Palaeobotany IOP NEWSLETTER 110 August 2016 CONTENTS FROM THE SECRETARY/TREASURER IPC XIV/IOPC X 2016 IOPC 2020 IOP MEMBERSHIP IOP EXECUTIVE COMMITTEE ELECTIONS IOP WEBMASTER POSITION WHAT HAPPENED TO THE OUPH COLLECTIONS? THE PALAEOBOTANY OF ITALY UPCOMING MEETINGS CALL FOR NEWS and NOTES The views expressed in the newsletter are those of its correspondents, and do not necessarily reflect the policy of IOP. Please send us your contributions for the next edition of our newsletter (June 2016) by M ay 30th, 2016. President: Johanna Eder-Kovar (G ermany) Vice Presidents: Bob Spicer (Great Britain), Harufumi Nishida (Japan), M ihai Popa (Romania) M embers at Large: Jun W ang (China), Hans Kerp (Germany), Alexej Herman (Russia) Secretary/Treasurer/Newsletter editor: M ike Dunn (USA) Conference/Congress Chair: Francisco de Assis Ribeiro dos Santos IOP Logo: The evolution of plant architecture (© by A. R. Hemsley) I OP 110 2 August 2016 FROM THE In addition, please send any issues that you think need to be addressed at the Business SECRETARY/TREASURER meeting. I will add those to the Agenda. Dear IOP Members, Respectfully, Mike I am happy to report, that IOP seems to be on track and ready for a new Executive Council to take over. The elections are IPC XIV/IOPC X 2016 progressing nicely and I will report the results in the September/October Newsletter. The one area that is still problematic is the webmaster position. We really to talk amongst ourselves, and find someone who is willing and able to do the job. -

The Jurassic Fossil Wood Diversity from Western Liaoning, NE China
Jiang et al. Journal of Palaeogeography (2019) 8:1 https://doi.org/10.1186/s42501-018-0018-y Journal of Palaeogeography RESEARCH Open Access The Jurassic fossil wood diversity from western Liaoning, NE China Zi-Kun Jiang1,2, Yong-Dong Wang2,3*, Ning Tian4,5, Ao-Wei Xie2,6, Wu Zhang7, Li-Qin Li2 and Min Huang1 Abstract Western Liaoning is a unique region in China that bears diverse types of Jurassic plants, including leaves, fern rhizomes, and wood, providing significant proxy for vegetation and palaeoenvironment reconstruction of the well-known Yanliao Flora in East Asia. In particular, the silicified wood is very abundant in the fossil Lagerstätte of the Jurassic Tiaojishan Formation in Beipiao, western Liaoning. Previous and recent systematic investigations documented a high diversity of the Jurassic wood assemblages. These assemblages are dominated by conifers, followed by cycads and ginkgoaleans. In total, about 30 species belonging to 21 genera of fossil wood have been recorded so far, which are represented by Cycadopsida, Ginkgopsida, Coniferopsida, and Gymnospermae incertae sedis. The evolutionary implications of several distinctive fossil wood taxa as well as palaeoclimate implications are summarized based on their anatomical structures and growth ring patterns. This work approaches the vegetation development and evolutionary significances of the wood taxa and their relatives, and provides clues for the further understanding of the diversity of the Jurassic Yanliao Flora in East Asia. Keywords: Fossil wood, Diversity, Evolution, Tiaojishan Formation, Jurassic 1 Introduction 2004;Wangetal.,2009). Among these localities, western Fossil floras are a significant record for the vegetation Liaoning is a well-known fossil Lagerstätte with diverse and for the palaeoenvironment reconstructions of the and well-preserved fossil plant foliages and wood (Zhang Mesozoic. -

Plant Species of Special Concern and Vascular Plant Flora of the National
Plant Species of Special Concern and Vascular Plant Flora of the National Elk Refuge Prepared for the US Fish and Wildlife Service National Elk Refuge By Walter Fertig Wyoming Natural Diversity Database The Nature Conservancy 1604 Grand Avenue Laramie, WY 82070 February 28, 1998 Acknowledgements I would like to thank the following individuals for their assistance with this project: Jim Ozenberger, ecologist with the Jackson Ranger District of Bridger-Teton National Forest, for guiding me in his canoe on Flat Creek and for providing aerial photographs and lodging; Jennifer Whipple, Yellowstone National Park botanist, for field assistance and help with field identification of rare Carex species; Dr. David Cooper of Colorado State University, for sharing field information from his 1994 studies; Dr. Ron Hartman and Ernie Nelson of the Rocky Mountain Herbarium, for providing access to unmounted collections by Michele Potkin and others from the National Elk Refuge; Dr. Anton Reznicek of the University of Michigan, for confirming the identification of several problematic Carex specimens; Dr. Robert Dorn for confirming the identification of several vegetative Salix specimens; and lastly Bruce Smith and the staff of the National Elk Refuge for providing funding and logistical support and for allowing me free rein to roam the refuge for plants. 2 Table of Contents Page Introduction . 6 Study Area . 6 Methods . 8 Results . 10 Vascular Plant Flora of the National Elk Refuge . 10 Plant Species of Special Concern . 10 Species Summaries . 23 Aster borealis . 24 Astragalus terminalis . 26 Carex buxbaumii . 28 Carex parryana var. parryana . 30 Carex sartwellii . 32 Carex scirpoidea var. scirpiformis . -

Lessons from 20 Years of Plant Genome Sequencing: an Unprecedented Resource in Need of More Diverse Representation
bioRxiv preprint doi: https://doi.org/10.1101/2021.05.31.446451; this version posted May 31, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Lessons from 20 years of plant genome sequencing: an unprecedented resource in need of more diverse representation Authors: Rose A. Marks1,2,3, Scott Hotaling4, Paul B. Frandsen5,6, and Robert VanBuren1,2 1. Department of Horticulture, Michigan State University, East Lansing, MI 48824, USA 2. Plant Resilience Institute, Michigan State University, East Lansing, MI 48824, USA 3. Department of Molecular and Cell Biology, University of Cape Town, Rondebosch 7701, South Africa 4. School of Biological Sciences, Washington State University, Pullman, WA, USA 5. Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT, USA 6. Data Science Lab, Smithsonian Institution, Washington, DC, USA Keywords: plants, embryophytes, genomics, colonialism, broadening participation Correspondence: Rose A. Marks, Department of Horticulture, Michigan State University, East Lansing, MI 48824, USA; Email: [email protected]; Phone: (603) 852-3190; ORCID iD: https://orcid.org/0000-0001-7102-5959 Abstract The field of plant genomics has grown rapidly in the past 20 years, leading to dramatic increases in both the quantity and quality of publicly available genomic resources. With an ever- expanding wealth of genomic data from an increasingly diverse set of taxa, unprecedented potential exists to better understand the evolution and genome biology of plants. -
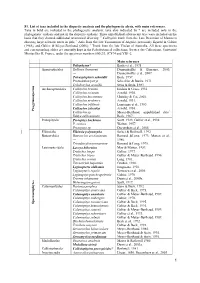
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

SYSTÉMATIQUE DES EMBRYOPHYTES Document Illustrant Plus Particulièrement Les Taxons Européens Version 4 Octobre 2007
Université Pierre et Marie Curie (PARIS 6) Préparation à l’agrégation SVSTU, secteur B SYSTÉMATIQUE DES EMBRYOPHYTES Document illustrant plus particulièrement les taxons européens version 4 octobre 2007 Catherine Reeb, Préparation agrégation SVSTU, UPMC Jean-Yves Dubuisson, Laboratoire Paléobotanique et Paléoécologie, équipe Paléodiversité, systématique et évolution des Embryophytes, UPMC Dessin de couverture : extrait d’un traité botanique tibétain Pour Garance Remerciements: merci à Jean et Monique Duperon pour les clés de détermination des familles d’Angiospermes, à A.M pour la reproduction de couverture, à Michaël Manuel et Eric Queinnec pour la partie I de l’introduction. Egalement à tous les illustrateurs qui ont autorisé l’utilisation de leurs documents mis en ligne sur Internet : Françoise Gantet, Prof. I. Foissner, Association Endemia de Nouvelle Calédonie. 1 Table des matières Introduction 3 Les données essentielles pour l’agrégation . 5 I Systématique générale des Embryophytes 6 I.1 Caractères des Embryophytes . 6 I.2 La place des Embryophytes au sein de la lignée verte . 7 I.3 Le groupe frère des Embryophytes . 7 I.4 Relations au sein des Embryophytes :les points acquis aujourd’hui . 8 I.4.1 Marchantiophytes, Bryophytes, Anthocérotophytes à la base des Embryophytes . 8 I.4.2 Les Trachéophytes ou plantes vasculaires, un groupe monophylétique . 9 I.4.3 Les Spermatophytes ou plantes à ovules, un groupe monophylétique . 9 I.5 Quelques points encore en discussion . 11 I.5.1 Position et relations des trois clades basaux (Bryophytes, Marchantiophytes et Anthocérotophytes) 11 I.5.2 Relations au sein des Spermatophytes :l’hypothèse "Gnepine" . 13 II Les principaux clades d’Embryophytes 15 II.1 MARCHANTIOPHYTA:Les Marchantiophytes ou Hépatiques . -

A New Genus Navipelta (Peltaspermales, Pteridospermae) from the Permian/Triassic Boundary Deposits of the Moscow Syneclise E
ISSN 0031-0301, Paleontological Journal, 2009, Vol. 43, No. 10, pp. 1262–1271. © Pleiades Publishing, Ltd., 2009. A New Genus Navipelta (Peltaspermales, Pteridospermae) from the Permian/Triassic Boundary Deposits of the Moscow Syneclise E. V. Karasev Borissiak Paleontological Institute of the Russian Academy of Sciences, 117997, Profsoyuznaya, 123, Moscow e-mail: [email protected] Received January 25, 2009 Abstract—A new genus of peltaspermalean ovuliferous organs Navipelta gen. nov. is described from the ter- restrial deposits of the Nedubrovo locality (village of Nedubrovo, Vologda Region, Russia), belonging to the base of Vetlugian Group (Upper Permian–Lower Triassic). Data on the anatomy of the peltate bilateral ovulif- erous organs are obtained for the first time. Vascular strands in the peltoid depart from that of a stalk and branch up to three times distally. Transfusion tissue around the vascular strands is well developed. The new genus had a system of radially arranged resin canals, broaden into large secretory cavities. Key words: Peltaspermaceae, ovuliferous organs, Peltaspermum, Autunia, Permian/Triassic boundary, Vetlu- gian Group, systematics. DOI: 10.1134/S0031030109100086 INTRODUCTION angium Zhao ex Gomankov et Meyen and Autuniopsis Poort et Kerp) or on the basis of their association with The family Peltaspermaceae attracts attention of different foliage (Peltaspermopsis buevichiae Goman- many researchers, because its members were the main kov et. Meyen and Meyenopteris, Poort et Kerp) component of the Late Permian Angaraland floras. (Gomankov and Meyen, 1986; Poort and Kerp, 1990). They escaped the global crisis on the Permian/Triassic boundary and transited in the Mesozoic, where domi- The morphology and epidermal structure of seed- nated during the Middle and Late Triassic of the North- bearing organs in the Peltaspermaceae were studied rel- ern as well as Southern hemispheres. -

Equisetalean Plant Remains from the Early to Middle Triassic of New South Wales, Australia
Records of the Australian Museum (2001) Vol. 53: 9–20. ISSN 0067-1975 Equisetalean Plant Remains from the Early to Middle Triassic of New South Wales, Australia W.B. KEITH HOLMES “Noonee Nyrang”, Gulgong Road, Wellington NSW 2820, Australia Honorary Research Fellow, Geology Department, University of New England, Armidale NSW 2351, Australia [email protected] Present address: National Botanical Institute, Private Bag X101, Pretoria, 0001, South Africa ABSTRACT. Equisetalean fossil plant remains of Early to Middle Triassic age from New South Wales are described. Robust and persistent nodal diaphragms composed of three zones; a broad central pith disc, a vascular cylinder and a cortical region surrounded by a sheath of conjoined leaf bases, are placed in Nododendron benolongensis n.sp. The new genus Townroviamites is erected for stems previously assigned to Phyllotheca brookvalensis which bear whorls of leaves forming a narrow basal sheath and the number of leaves matches the number of vascular bundles. Finely striated stems bearing leaf whorls consisting of several foliar lobes each formed from four to seven linear conjoined leaves are described as Paraschizoneura jonesii n.sp. Doubts are raised about the presence of the common Permian Gondwanan sphenophyte species Phyllotheca australis and the Northern Hemisphere genus Neocalamites in Middle Triassic floras of Gondwana. HOLMES, W.B. KEITH, 2001. Equisetalean plant remains from the Early to Middle Triassic of New South Wales, Australia. Records of the Australian Museum 53(1): 9–20. The plant Phylum Sphenophyta, which includes the Permian Period, the increasing aridity and decline in the equisetaleans, commonly known as “horse-tails” or vegetation of northern Pangaea was in contrast to that in “scouring rushes”, first appeared during the Devonian southern Pangaea—Gondwana—where flourishing swamp Period (Taylor & Taylor, 1993). -

The Big Bloom—How Flowering Plants Changed the World
The Big Bloom—How Flowering Plants Changed the World Written by Michael Klesius Republished from the pages of National Geographic magazine -- July 2002 In the summer of 1973 sunflowers appeared in my father's vegetable garden. They seemed to sprout overnight in a few rows he had lent that year to new neighbors from California. Only six years old at the time, I was at first put off by these garish plants. Such strange and vibrant flowers seemed out of place among the respectable beans, peppers, spinach, and other vegetables we had always grown. Gradually, however, the brilliance of the sunflowers won me over. Their fiery halos relieved the green monotone that by late summer ruled the garden. I marveled at birds that clung upside down to the shaggy, gold disks, wings fluttering, looting the seeds. Sunflowers defined flowers for me that summer and changed my view of the world. Flowers have a way of doing that. They began changing the way the world looked almost as soon as they appeared on Earth about 130 million years ago, during the Cretaceous period. That's relatively recent in geologic time: If all Earth's history were compressed into an hour, flowering plants would exist for only the last 90 seconds. But once they took firm root about 100 million years ago, they swiftly diversified in an explosion of varieties that established most of the flowering plant families of the modern world. Today flowering plant species outnumber by twenty to one those of ferns and cone-bearing trees, or conifers, which had thrived for 200 million years before the first bloom appeared. -

Life in the End-Permian Dead Zone
Life in the end-Permian dead zone Cindy V. Looy*†, Richard J. Twitchett‡, David L. Dilcher§, Johanna H. A. Van Konijnenburg-Van Cittert*, and Henk Visscher* *Laboratory of Palaeobotany and Palynology, Utrecht University, Budapestlaan 4, 3584 CD Utrecht, The Netherlands; ‡Department of Earth Sciences, University of Southern California, Los Angeles, CA 90089-0740; and §Paleobotany Laboratory, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611 Contributed by David L. Dilcher, May 1, 2001 The fossil record of land plants is an obvious source of information ecological crisis. On the basis of palynological data from a ‘‘dead on the dynamics of mass extinctions in the geological past. In zone’’ in a Permian–Triassic (P-Tr) transition sequence from conjunction with the end-Permian ecological crisis, Ϸ250 million East Greenland, in this paper we document evidence of non- years ago, palynological data from East Greenland reveal some equilibrium vegetation dynamics resulting in selective but time- unanticipated patterns. We document the significant time lag delayed extinctions among woody gymnosperms. between terrestrial ecosystem collapse and selective extinction among characteristic Late Permian plants. Furthermore, ecological The End-Permian ‘‘Dead Zone’’ crisis resulted in an initial increase in plant diversity, instead of a Latest Permian and earliest Triassic sediments in East Green- decrease. Paradoxically, these floral patterns correspond to a land (Fig. 1) are represented by the upper part of the Schuchert ‘‘dead zone’’ in the end-Permian faunal record, characterized by a Dal Formation and the overlying Wordie Creek Formation. The paucity of marine invertebrate megafossils. The time-delayed, predominantly fine-grained siliciclastic sediments of these for- end-Permian plant extinctions resemble modeled ‘‘extinction mations were deposited in a narrow, elongate, shallow-marine debt’’ responses of multispecies metapopulations to progressive basin.