M23 Tree History Workbook Pub 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contents Part III. the LAST FIFTY THOUSAND YEARS
STATE OF MICHIGAN Insects ................................................................... 60 DEPARTMENT OF CONSERVATION Worms And Others ................................................ 61 P. J. Hoffmaster, Director The Pleasant Peninsulas .............................................62 Man and his Towns ......................................................69 THEY NEED NOT VANISH A DISCUSSION OF THE NATURAL RESOURCES In Conclusion .................................................................71 OF MICHIGAN Part III. THE LAST FIFTY THOUSAND YEARS "THE GOOD EARTH" Edited by HELEN M. MARTIN ir, sunlight, water, and soil are essential for the Acontinuance of life—plant, animal, and human life, from contributions by on the earth. Of these four basic requirements, the soil Shirley W. Allen, Geo. C. S. Benson, University of is most directly subject to the care and management of Michigan man. However, the soil has frequently been the object of man's most careless use and abuse. It is, therefore, Stannard B. Bergquist, L. R. Schoenmann, H. C. most fitting that the eminent soil scientist, A. F. Beeskaw, J. H. Kraemer, W. F. Morofsky, J. A. Porter, E. Gustafson, should begin his book on soils and soil C. Sackrider, Michigan State College management with: G. S. Mclntire, H. M. Martin, O. F. Poindexter, C. F. "During his existence upon the earth, man has depended upon Welch, Department of Conservation the soil, either directly or indirectly, for the production of the materials used by him for food and clothing and, in part, for the M. G. Adams, Stream Control Commission production of those used for fuel and shelter as well. Grains, Frank DuMond, Public Museum, Grand Rapids fruits, and vegetables that serve him as food grow directly on the soil. Cotton and flax yield materials that are made into Lynn Heatley, High School, Midland. -

History and Philosophy of Systematic Biology
History and Philosophy of Systematic Biology Bock, W. J. (1973) Philosophical foundations of classical evolutionary classification Systematic Zoology 22: 375-392 Part of a general symposium on "Contemporary Systematic Philosophies," there are some other interesting papers here. Brower, A. V. Z. (2000) Evolution Is Not a Necessary Assumption of Cladistics Cladistics 16: 143- 154 Dayrat, Benoit (2005) Ancestor-descendant relationships and the reconstruction of the Tree of Lif Paleobiology 31: 347-353 Donoghue, M.J. and J.W. Kadereit (1992) Walter Zimmermann and the growth of phylogenetic theory Systematic Biology 41: 74-84 Faith, D. P. and J. W. H. Trueman (2001) Towards an inclusive philosophy for phylogenetic inference Systematic Biology 50: 331-350 Gaffney, E. S. (1979) An introduction to the logic of phylogeny reconstruction, pp. 79-111 in Cracraft, J. and N. Eldredge (eds.) Phylogenetic Analysis and Paleontology Columbia University Press, New York. Gilmour, J. S. L. (1940) Taxonomy and philosophy, pp. 461-474 in J. Huxley (ed.) The New Systematics Oxford Hull, D. L. (1978) A matter of individuality Phil. of Science 45: 335-360 Hull, D. L. (1978) The principles of biological classification: the use and abuse of philosophy Hull, D. L. (1984) Cladistic theory: hypotheses that blur and grow, pp. 5-23 in T. Duncan and T. F. Stuessy (eds.) Cladistics: Perspectives on the Reconstruction of Evolutionary History Columbia University Press, New York * Hull, D. L. (1988) Science as a process: an evolutionary account of the social and conceptual development of science University of Chicago Press. An already classic work on the recent, violent history of systematics; used as data for Hull's general theories about scientific change. -

RI Equisetopsida and Lycopodiopsida.Indd
IIntroductionntroduction byby FFrancisrancis UnderwoodUnderwood Rhode Island Equisetopsida, Lycopodiopsida and Isoetopsida Special Th anks to the following for giving permission for the use their images. Robbin Moran New York Botanical Garden George Yatskievych and Ann Larson Missouri Botanical Garden Jan De Laet, plantsystematics.org Th is pdf is a companion publication to Rhode Island Equisetopsida, Lycopodiopsida & Isoetopsida at among-ri-wildfl owers.org Th e Elfi n Press 2016 Introduction Formerly known as fern allies, Horsetails, Club-mosses, Fir-mosses, Spike-mosses and Quillworts are plants that have an alternate generation life-cycle similar to ferns, having both sporophyte and gametophyte stages. Equisetopsida Horsetails date from the Devonian period (416 to 359 million years ago) in earth’s history where they were trees up to 110 feet in height and helped to form the coal deposits of the Carboniferous period. Only one genus has survived to modern times (Equisetum). Horsetails Horsetails (Equisetum) have jointed stems with whorls of thin narrow leaves. In the sporophyte stage, they have a sterile and fertile form. Th ey produce only one type of spore. While the gametophytes produced from the spores appear to be plentiful, the successful reproduction of the sporophyte form is low with most Horsetails reproducing vegetatively. Lycopodiopsida Lycopodiopsida includes the clubmosses (Dendrolycopodium, Diphasiastrum, Lycopodiella, Lycopodium , Spinulum) and Fir-mosses (Huperzia) Clubmosses Clubmosses are evergreen plants that produce only microspores that develop into a gametophyte capable of producing both sperm and egg cells. Club-mosses can produce the spores either in leaf axils or at the top of their stems. Th e spore capsules form in a cone-like structures (strobili) at the top of the plants. -

Fossils and Plant Phylogeny1
American Journal of Botany 91(10): 1683±1699. 2004. FOSSILS AND PLANT PHYLOGENY1 PETER R. CRANE,2,5 PATRICK HERENDEEN,3 AND ELSE MARIE FRIIS4 2Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK; 3Department of Biological Sciences, The George Washington University, Washington DC 20052 USA; 4Department of Palaeobotany, Swedish Museum of Natural History, Box 50007, S-104 05 Stockholm, Sweden Developing a detailed estimate of plant phylogeny is the key ®rst step toward a more sophisticated and particularized understanding of plant evolution. At many levels in the hierarchy of plant life, it will be impossible to develop an adequate understanding of plant phylogeny without taking into account the additional diversity provided by fossil plants. This is especially the case for relatively deep divergences among extant lineages that have a long evolutionary history and in which much of the relevant diversity has been lost by extinction. In such circumstances, attempts to integrate data and interpretations from extant and fossil plants stand the best chance of success. For this to be possible, what will be required is meticulous and thorough descriptions of fossil material, thoughtful and rigorous analysis of characters, and careful comparison of extant and fossil taxa, as a basis for determining their systematic relationships. Key words: angiosperms; fossils; paleobotany; phylogeny; spermatophytes; tracheophytes. Most biological processes, such as reproduction or growth distic context, neither fossils nor their stratigraphic position and development, can only be studied directly or manipulated have any special role in inferring phylogeny, and although experimentally using living organisms. Nevertheless, much of more complex models have been developed (see Fisher, 1994; what we have inferred about the large-scale processes of plant Huelsenbeck, 1994), these have not been widely adopted. -

Chromosome Numbers in Gymnosperms - an Update
Rastogi and Ohri . Silvae Genetica (2020) 69, 13 - 19 13 Chromosome Numbers in Gymnosperms - An Update Shubhi Rastogi and Deepak Ohri Amity Institute of Biotechnology, Research Cell, Amity University Uttar Pradesh, Lucknow Campus, Malhaur (Near Railway Station), P.O. Chinhat, Luc know-226028 (U.P.) * Corresponding author: Deepak Ohri, E mail: [email protected], [email protected] Abstract still some controversy with regard to a monophyletic or para- phyletic origin of the gymnosperms (Hill 2005). Recently they The present report is based on a cytological data base on 614 have been classified into four subclasses Cycadidae, Ginkgoi- (56.0 %) of the total 1104 recognized species and 82 (90.0 %) of dae, Gnetidae and Pinidae under the class Equisetopsida the 88 recognized genera of gymnosperms. Family Cycada- (Chase and Reveal 2009) comprising 12 families and 83 genera ceae and many genera of Zamiaceae show intrageneric unifor- (Christenhusz et al. 2011) and 88 genera with 1104 recognized mity of somatic numbers, the genus Zamia is represented by a species according to the Plant List (www.theplantlist.org). The range of number from 2n=16-28. Ginkgo, Welwitschia and Gen- validity of accepted name of each taxa and the total number of tum show 2n=24, 2n=42, and 2n=44 respectively. Ephedra species in each genus has been checked from the Plant List shows a range of polyploidy from 2x-8x based on n=7. The (www.theplantlist.org). The chromosome numbers of 688 taxa family Pinaceae as a whole shows 2n=24except for Pseudolarix arranged according to the recent classification (Christenhusz and Pseudotsuga with 2n=44 and 2n=26 respectively. -

Supplementary Material Local and Expert
10.1071/PC14920_AC CSIRO 2015 Pacific Conservation Biology 21 (3), 214-219 Supplementary material Local and expert knowledge improve conservation assessment of rare and iconic Fijian tree species Gunnar KeppelA,F, Alifereti NaikatiniB, Isaac A. RoundsC, Robert L. PresseyD, and Nunia T. ThomasE ASchool of Natural and Built Environments and Barbara Hardy Institute, University of South Australia, Mawson Lakes Campus, GPO Box 2471, Adelaide, SA 5001, Australia. BSouth Pacific Regional Herbarium, University of the South Pacific CConservation International, Suva, Fiji DAustralian Research Council Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, QLD 4811 Australia. ENatureFiji-MareqetiViti, 14 Hamilton-Beattie Street, Suva, Fiji FCorresponding author. Email: [email protected] Part 1: Overview of conservation status for each study species before this study. Acmopyle sahniana Buchholz & N.E. Gray (Podocarpaceae) is a rare conifer to 12 m tall, previously only reported from forested mountain ridges from central Viti Levu (Bush and Doyle 1997, Thomas 2013a). A detailed survey of the species recorded a total of 46 adult and 17 juvenile trees in 2 subpopulations (Bush 1997). A recent (2011) assessment reported another subpopulation near Fiji’s highest mountain, Mt. Tomanivi, and estimated the total size of that subpopulation at <100 mature individuals (Thomas 2013a). The species is listed as critically endangered (CR), based on small population size and low area of occupancy (<10 km2) (Thomas 2013a). Cynometra falcata A.Gray (Leguminosae) is reported as a slender tree to 4 m in height that until recently had only been known from two locations, one on Vanua Levu and another on Viti Levu (Smith 1985, WCMC 1998). -
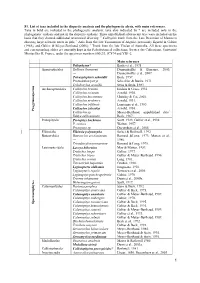
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

SYSTÉMATIQUE DES EMBRYOPHYTES Document Illustrant Plus Particulièrement Les Taxons Européens Version 4 Octobre 2007
Université Pierre et Marie Curie (PARIS 6) Préparation à l’agrégation SVSTU, secteur B SYSTÉMATIQUE DES EMBRYOPHYTES Document illustrant plus particulièrement les taxons européens version 4 octobre 2007 Catherine Reeb, Préparation agrégation SVSTU, UPMC Jean-Yves Dubuisson, Laboratoire Paléobotanique et Paléoécologie, équipe Paléodiversité, systématique et évolution des Embryophytes, UPMC Dessin de couverture : extrait d’un traité botanique tibétain Pour Garance Remerciements: merci à Jean et Monique Duperon pour les clés de détermination des familles d’Angiospermes, à A.M pour la reproduction de couverture, à Michaël Manuel et Eric Queinnec pour la partie I de l’introduction. Egalement à tous les illustrateurs qui ont autorisé l’utilisation de leurs documents mis en ligne sur Internet : Françoise Gantet, Prof. I. Foissner, Association Endemia de Nouvelle Calédonie. 1 Table des matières Introduction 3 Les données essentielles pour l’agrégation . 5 I Systématique générale des Embryophytes 6 I.1 Caractères des Embryophytes . 6 I.2 La place des Embryophytes au sein de la lignée verte . 7 I.3 Le groupe frère des Embryophytes . 7 I.4 Relations au sein des Embryophytes :les points acquis aujourd’hui . 8 I.4.1 Marchantiophytes, Bryophytes, Anthocérotophytes à la base des Embryophytes . 8 I.4.2 Les Trachéophytes ou plantes vasculaires, un groupe monophylétique . 9 I.4.3 Les Spermatophytes ou plantes à ovules, un groupe monophylétique . 9 I.5 Quelques points encore en discussion . 11 I.5.1 Position et relations des trois clades basaux (Bryophytes, Marchantiophytes et Anthocérotophytes) 11 I.5.2 Relations au sein des Spermatophytes :l’hypothèse "Gnepine" . 13 II Les principaux clades d’Embryophytes 15 II.1 MARCHANTIOPHYTA:Les Marchantiophytes ou Hépatiques . -

The Cycad Newsletter
Manuscript for: The Cycad Newsletter Title: A rescue, propagation, and reintroduction program for one of the most endangered lineage of cycads, Chigua in Northwestern Colombia Authors: Cristina López-Gallego1 Norberto López Alvarez2 Authors affiliations: 1 Instituto de Biología, Universidad de Antioquia, Colombia 2 Fundacion Biozoo, Córdoba, Colombia Correspondence author: Cristina López-Gallego E-mail: [email protected] Address: Kra. 75A #32A-26, Medellin, Colombia Phone: 574-238-6112 Chigua was first collected as an unicate by Francis Pennell in 1918. It was not until 1986 that a population conforming to Pennell's collection was relocated by the Colombian botanist Rodrigo Bernal. In 1990 Dennis Stevenson described two species of the new genus Chigua based on collections made by him, Knut Norstog, and the Colombian botanist Padre Sergio Restrepo in the only known locality for the two species in northwestern Colombia. By the time the next collections were made at the end of the 1990s (by Colombian botanists Alvaro Idárraga, Carlos A. Gutiérrez, Antonio Duque, and Cristina López-Gallego), the known population used for species descriptions had mostly disappeared because of habitat destruction and only a few scattered individuals were observed near the type locality. The two species of Chigua resemble some acaulous Zamia species, e.g. Zamia melanorrhachis D. Stev., in their overall morphology: a small subterraneous rhizome, few armed leaves with elliptic and papery toothed leaflets, and small cones with peltate unornamented sporophylls, and ovoid reddish seeds; but the presence of a central conspicuous vein distinguishes Chigua species from the rest of the Zamia lineage. The two species of Chigua can be separated by the shape of the mid-leaf leaflets, with C. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

Embryophytic Sporophytes in the Rhynie and Windyfield Cherts
Transactions of the Royal Society of Edinburgh: Earth Sciences http://journals.cambridge.org/TRE Additional services for Transactions of the Royal Society of Edinburgh: Earth Sciences: Email alerts: Click here Subscriptions: Click here Commercial reprints: Click here Terms of use : Click here Embryophytic sporophytes in the Rhynie and Windyeld cherts Dianne Edwards Transactions of the Royal Society of Edinburgh: Earth Sciences / Volume 94 / Issue 04 / December 2003, pp 397 - 410 DOI: 10.1017/S0263593300000778, Published online: 26 July 2007 Link to this article: http://journals.cambridge.org/abstract_S0263593300000778 How to cite this article: Dianne Edwards (2003). Embryophytic sporophytes in the Rhynie and Windyeld cherts. Transactions of the Royal Society of Edinburgh: Earth Sciences, 94, pp 397-410 doi:10.1017/S0263593300000778 Request Permissions : Click here Downloaded from http://journals.cambridge.org/TRE, IP address: 131.251.254.13 on 25 Feb 2014 Transactions of the Royal Society of Edinburgh: Earth Sciences, 94, 397–410, 2004 (for 2003) Embryophytic sporophytes in the Rhynie and Windyfield cherts Dianne Edwards ABSTRACT: Brief descriptions and comments on relationships are given for the seven embryo- phytic sporophytes in the cherts at Rhynie, Aberdeenshire, Scotland. They are Rhynia gwynne- vaughanii Kidston & Lang, Aglaophyton major D. S. Edwards, Horneophyton lignieri Barghoorn & Darrah, Asteroxylon mackiei Kidston & Lang, Nothia aphylla Lyon ex Høeg, Trichopherophyton teuchansii Lyon & Edwards and Ventarura lyonii Powell, Edwards & Trewin. The superb preserva- tion of the silica permineralisations produced in the hot spring environment provides remarkable insights into the anatomy of early land plants which are not available from compression fossils and other modes of permineralisation. -

Weathering Behaviour of Cunninghamia Lanceolata (Lamb.) Hook
Article Weathering Behaviour of Cunninghamia lanceolata (Lamb.) Hook. under Natural Conditions Xinjie Cui 1 and Junji Matsumura 2,* 1 Graduate School of Bioresource and Bioenvironmental Sciences, Faculty of Agriculture, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan; [email protected] 2 Laboratory of Wood Science, Faculty of Agriculture, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan * Correspondence: [email protected]; Tel.: +81-092-802-4656 Received: 18 July 2020; Accepted: 10 December 2020; Published: 14 December 2020 Abstract: Information on the weathering behaviour of Cunninghamia lanceolata (Lamb.) Hook. is needed to provide references for wood weatherproof pre-treatment and to improve wood utilization. Therefore, this study was conducted to understand the variation in the intrinsic weathering behaviour of Cunninghamia lanceolata (Chinese fir) under natural conditions. Wood samples from 15 Cunninghamia lanceolata trees aged 26–30 years old were used. The structural degradation and discoloration of wood surfaces before and after exposure were compared. The results show that the weathering behaviour of wood was weakened from heartwood to sapwood and enhanced from the bottom to the top. This study provided information for weatherability research and improved wood utilization of Cunninghamia lanceolata. Keywords: Cunninghamia lanceolata; weathering; density; colour change; wood structure 1. Introduction Cunninghamia lanceolata (Lamb.) Hook. is a member of the family Cupressaceae. It is an evergreen tree that can grow up to 50 m in height and over 3 m in diameter. It forms mixed broad-leaved forests or small, pure stands, rocky hillsides, roadsides, with altitudes ranging from 200 to 2800 m [1].