This Article Appeared in a Journal Published by Elsevier. the Attached
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
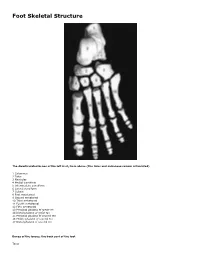
Skeletal Foot Structure
Foot Skeletal Structure The disarticulated bones of the left foot, from above (The talus and calcaneus remain articulated) 1 Calcaneus 2 Talus 3 Navicular 4 Medial cuneiform 5 Intermediate cuneiform 6 Lateral cuneiform 7 Cuboid 8 First metatarsal 9 Second metatarsal 10 Third metatarsal 11 Fourth metatarsal 12 Fifth metatarsal 13 Proximal phalanx of great toe 14 Distal phalanx of great toe 15 Proximal phalanx of second toe 16 Middle phalanx of second toe 17 Distal phalanx of second toe Bones of the tarsus, the back part of the foot Talus Calcaneus Navicular bone Cuboid bone Medial, intermediate and lateral cuneiform bones Bones of the metatarsus, the forepart of the foot First to fifth metatarsal bones (numbered from the medial side) Bones of the toes or digits Phalanges -- a proximal and a distal phalanx for the great toe; proximal, middle and distal phalanges for the second to fifth toes Sesamoid bones Two always present in the tendons of flexor hallucis brevis Origin and meaning of some terms associated with the foot Tibia: Latin for a flute or pipe; the shin bone has a fanciful resemblance to this wind instrument. Fibula: Latin for a pin or skewer; the long thin bone of the leg. Adjective fibular or peroneal, which is from the Greek for pin. Tarsus: Greek for a wicker frame; the basic framework for the back of the foot. Metatarsus: Greek for beyond the tarsus; the forepart of the foot. Talus (astragalus): Latin (Greek) for one of a set of dice; viewed from above the main part of the talus has a rather square appearance. -

Rethinking the Evolution of the Human Foot: Insights from Experimental Research Nicholas B
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174425. doi:10.1242/jeb.174425 REVIEW Rethinking the evolution of the human foot: insights from experimental research Nicholas B. Holowka* and Daniel E. Lieberman* ABSTRACT presumably owing to their lack of arches and mobile midfoot joints Adaptive explanations for modern human foot anatomy have long for enhanced prehensility in arboreal locomotion (see Glossary; fascinated evolutionary biologists because of the dramatic differences Fig. 1B) (DeSilva, 2010; Elftman and Manter, 1935a). Other studies between our feet and those of our closest living relatives, the great have documented how great apes use their long toes, opposable apes. Morphological features, including hallucal opposability, toe halluces and mobile ankles for grasping arboreal supports (DeSilva, length and the longitudinal arch, have traditionally been used to 2009; Holowka et al., 2017a; Morton, 1924). These observations dichotomize human and great ape feet as being adapted for bipedal underlie what has become a consensus model of human foot walking and arboreal locomotion, respectively. However, recent evolution: that selection for bipedal walking came at the expense of biomechanical models of human foot function and experimental arboreal locomotor capabilities, resulting in a dichotomy between investigations of great ape locomotion have undermined this simple human and great ape foot anatomy and function. According to this dichotomy. Here, we review this research, focusing on the way of thinking, anatomical features of the foot characteristic of biomechanics of foot strike, push-off and elastic energy storage in great apes are assumed to represent adaptations for arboreal the foot, and show that humans and great apes share some behavior, and those unique to humans are assumed to be related underappreciated, surprising similarities in foot function, such as to bipedal walking. -

5Th Metatarsal Fracture
FIFTH METATARSAL FRACTURES Todd Gothelf MD (USA), FRACS, FAAOS, Dip. ABOS Foot, Ankle, Shoulder Surgeon Orthopaedic You have been diagnosed with a fracture of the fifth metatarsal bone. Surgeons This tyPe of fracture usually occurs when the ankle suddenly rolls inward. When the ankle rolls, a tendon that is attached to the fifth metatarsal bone is J. Goldberg stretched. Because the bone is weaker than the tendon, the bone cracks first. A. Turnbull R. Pattinson A. Loefler All bones heal in a different way when they break. This is esPecially true J. Negrine of the fifth metatarsal bone. In addition, the blood suPPly varies to different I. PoPoff areas, making it a lot harder for some fractures to heal without helP. Below are D. Sher descriPtions of the main Patterns of fractures of the fifth metatarsal fractures T. Gothelf and treatments for each. Sports Physicians FIFTH METATARSAL AVULSION FRACTURE J. Best This fracture Pattern occurs at the tiP of the bone (figure 1). These M. Cusi fractures have a very high rate of healing and require little Protection. Weight P. Annett on the foot is allowed as soon as the Patient is comfortable. While crutches may helP initially, walking without them is allowed. I Prefer to Place Patients in a walking boot, as it allows for more comfortable walking and Protects the foot from further injury. RICE treatment is initiated. Pain should be exPected to diminish over the first four weeks, but may not comPletely go away for several months. Follow-uP radiographs are not necessary if the Pain resolves as exPected. -

Analysis of the Talus and Calcaneus Bones from the Poole-Rose Ossuary
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2005 Analysis of the talus and calcaneus bones from the Poole-Rose Ossuary: a Late Woodland burial site in Ontario, Canada Adrienne Elizabeth Penney Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Social and Behavioral Sciences Commons Recommended Citation Penney, Adrienne Elizabeth, "Analysis of the talus and calcaneus bones from the Poole-Rose Ossuary: a Late Woodland burial site in Ontario, Canada" (2005). LSU Master's Theses. 3114. https://digitalcommons.lsu.edu/gradschool_theses/3114 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ANALYSIS OF THE TALUS AND CALCANEUS BONES FROM THE POOLE-ROSE OSSUARY: A LATE WOODLAND BURIAL SITE IN ONTARIO, CANADA A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Arts in The Department of Geography and Anthropology by Adrienne Elizabeth Penney B.A. University of Evansville, 2003 August 2005 ACKNOWLEDGEMENTS I appreciate the cooperation of the Alderville First Nation, and especially Nora Bothwell, for allowing the study of the valuable Poole-Rose collection. I would like to thank Ms. Mary H. Manhein, my advisor, for all of her time, encouragement, and assistance. -

Management of Intra-Articular Fractures of the Calcaneus: Introducing a New Locking Plate
Shafa Ortho J. 2018 November; 5(4):e7991. doi: 10.5812/soj.7991. Published online 2018 October 9. Letter Management of Intra-Articular Fractures of the Calcaneus: Introducing a New Locking Plate Bijan Valiollahi 1, * and Mostafa Salariyeh 1 1Bone and Joint Reconstruction Research Center, Shafa Orthopedic Hospital, Iran University of Medical Sciences, Tehran, Iran *Corresponding author: Bone and Joint Reconstruction Research Center, Shafa Orthopedic Hospital, Iran University of Medical Sciences, Tehran, Iran. Email: [email protected] Received 2016 October 31; Revised 2018 September 22; Accepted 2018 September 27. Keywords: Calcaneus Fracture, New Plate, Sinus Tarsal Approach Dear Editor, displaced intra-articular fractures involving the posterior Fracture of the calcaneus is usually a challenging prob- facet and is ideally performed within three weeks of in- lem for orthopedic surgeons and patients. Calcaneal frac- jury. Beyond this time, separation of the fragments be- tures are the most common of tarsal bone fractures, and comes more challenging. To achieve an adequate reduc- most of them are displaced intra-articular (nearly 75%) frac- tion, surgery must be performed after soft tissue swelling tures, usually resulting from falling or motor vehicle acci- diminishes (2). dents (1). Full diminishing of soft tissue edema is demonstrated Most patients with calcaneal fractures are usually by a positive wrinkle test, indicating that surgical interven- young men in their prime working years, which results in tion may be performed safely. For the extensile lateral ap- a significant loss of economic productivity. Although sur- proach, a good skin with positive wrinkle test is needed. gical techniques and fixation implants have generally im- Concerns about high wound complication rates in the ex- proved functional outcomes, there is vast controversy as to tensile lateral approach have prompted some to improve the management of these highly complex injuries. -

Autogenous Calcaneal Dowel Grafting Jones Fractures: Technique and Case Study Edward G
Autogenous Calcaneal Dowel Grafting Jones Fractures: Technique and Case Study Edward G. Blahous JR, DPM, FACFAS, Richard T. Bouché, DPM, FACFAS, Amol Saxena, DPM, FACFAS, FAAPSM, Chad Seidenstricker, DPM, PGY-3 A trephine is now used to obtain a bone graft plug, which will be used as an inlay graft to be placed into reamed hole. To assure a tight custom fit trephine diameter should be Introduction 1 mm greater than the reamer size. The authors’ prefer use of bone graft procured Many foot and ankle surgeons elect to treat the proximal fifth metatarsal base from the lateral calcaneal wall (Fig 6). The bone plug graft is then gently aligned and fractures near the metaphyseal-diaphyseal junction by surgical repair due to tamped into place (Fig 8). unpredictable outcomes with nonoperative management. Intramedullary fixation (IM) is the current “gold standard” and has high union rate [1]. However, there are several reports of complications with the IM technique. Notably, refracture, prominent hardware, and slow time to fracture healing [2-5]. This publication aims to describe a novel technique to treat the problematic Jones fracture. Figure 10. Intraoperative anteroposterior fluoroscopic Figure 11. Intraoperative lateral fluoroscopic view Figure 6. Incision placement with trephine in Figure 5. Visualization of recipient hole view of fracture site with graft and fixation in place. of fracture site with graft and fixation in place. Surgical Technique [6] place for safe exposure of lateral calcaneal through fracture site. The patient is placed in a lateral decubitus position with ipsilateral side facing wall. superiorly. A dorsolateral incision is made centered over the fifth metatarsal base Discussion fracture site. -

Foot and Ankle Injuries
Foot and Ankle Injuries Today’s lecture can be viewed at Learning in Athletics the following URL address: We most likely will not get “All of life should be a through all these slides today, learning experience, not Thomas W. Kaminski, PhD, ATC, http://sites.udel.edu/chshowever the presentation- atep/lectures/is just for the trivial FNATA, FACSM, RFSA thorough and complete and will reasons but because by Professor make for an excellent study continuing the learning Director of Athletic Training Education guide for the BOC process, we are challenging our brain University of Delaware examination! Good Luck! and therefore building brain circuitry” Arnold Scheibel Athletic Training & Sports Laboratory Manual UD is located here! A First State to accompany Fact! Health Care: The Journal for the Rehabilitation Techniques in Sports Medicine Delaware is 96 miles Practicing Clinician and Athletic Training long and varies from th 9 to 35 miles in 6 Edition width. http://www.healio.com /journals/atshc https://www.healio.com/books/athletic- training/%7b927168ac-1da2-422c-b98e- ae9013d4e15b%7d/rehabilitation-techniques-for- sports-medicine-and-athletic-training-sixth-edition Perhaps ATC’s Need to Incorporate Lower Extremity ~ Foot this Into Their Assessment Scheme? Anatomical Review Cyriax- “Diagnosis is only a matter of applying one’s anatomy” 1 Clinical PEARL: Lower Extremity ~ Foot Navicular Palpation Lower Extremity ~ Foot • Palpated 2-3 cm anteroinferior to the medial malleolus • More prominent with foot adducted Radiographically Viewed The Ankle Mortise Articulations Ankle Joint (Medial View) 1. Fibula 2. Tibia 3. Ankle jointChallenge 4. Promontoryyourself of tibia to 5. Trochlear surface of talus 6. -

Charcot Fracture in the Calcaneus After Total Knee Arthroplasty: a Case Report
Case Report Journal of Orthopaedic Case Reports 2016 Nov-Dec: 6(5):92-95 Charcot Fracture in the Calcaneus after Total Knee Arthroplasty: A Case Report Hirokazu Takai1, Katsuhiko Kiyota1, Nobutake Nakane1, Tomoki Takahashi1 What to Learn from this Article? Calcaneal stress fracture may occur after total knee arthroplasty in patients with neuropathic disorders such as a Charcot joint disease. Abstract Introduction: Only a few reports have described calcaneus fractures after total knee arthroplasty (TKA). Therefore, in this report, we describe a case of calcaneal avulsion fracture that occurred 5 weeks after a TKA in a relatively young male patient with syphilis. Case Report: A 63-year-old man with syphilis had Charcot joint of the right knee. The patient developed severe varus deformity and contracture and experienced severe pain in the knee. TKA was performed to alleviate the pain and improve the patient’s gait. The patient noticed slight heel pain 4 weeks after the TKA, and a calcaneus avulsion fracture in the ipsilateral foot was diagnosed without any trauma 1 week later. Open reduction internal fixation was performed with cannulated cancellous screws and a cerclage wire. 3 weeks after the surgery, partial weight bearing was permitted in an orthotic device. Full weight-bearing was allowed at 7 weeks after surgery. The surgical wounds healed without complications. The calcaneus fracture successfully achieved bone union with appropriate surgical intervention and aftercare. Conclusion: The previous studies have shown that calcaneus stress fracture may occur in elderly osteoporotic women after TKA. Patients with peripheral neuropathy may develop a Charcot fracture after minimal trauma because of decreased protective sensation, even if the patient is a relatively young man without osteoporosis. -

Does the Calcaneus Serve As Hypomochlion Within the Lower Limb by a Myofascial Connection?—A Systematic Review
life Review Does the Calcaneus Serve as Hypomochlion within the Lower Limb by a Myofascial Connection?—A Systematic Review Luise Weinrich 1, Melissa Paraskevaidis 1, Robert Schleip 2,3 , Alison N. Agres 4 and Serafeim Tsitsilonis 1,* 1 Center for Musculoskeletal Surgery, Charité—Universitätsmedizin Berlin, 13353 Berlin, Germany; [email protected] (L.W.); [email protected] (M.P.) 2 Technische Universität München, 80333 München, Germany; [email protected] 3 Diploma University of Applied Sciences, 37242 Bad Sooden-Allendorf, Germany 4 Julius Wolff Institute, Berlin Institute of Health and Charité—Universitätsmedizin Berlin, 13353 Berlin, Germany; [email protected] * Correspondence: [email protected] Abstract: (1) Background: Clinical approaches have depicted interconnectivity between the Achilles tendon and the plantar fascia. This concept has been applied in rehabilitation, prevention, and in conservative management plans, yet potential anatomical and histological connection is not fully understood. (2) Objective: To explore the possible explanation that the calcaneus acts as a hypomochlion. (3) Methods: 2 databases (Pubmed and Livivo) were searched and studies, including those that examined the relationship of the calcaneus to the Achilles tendon and plantar fascia and its biomechanical role. The included studies highlighted either the anatomical, histological, or biomechanical aspect of the lower limb. (4) Results: Seventeen studies were included. Some studies depicted an anatomical connection that slowly declines with age. Others mention a histological Citation: Weinrich, L.; similarity and continuity via the paratenon, while a few papers have brought forward mechanical Paraskevaidis, M.; Schleip, R.; reasoning. (5) Conclusion: The concept of the calcaneus acting as a fulcrum in the lower limb can Agres, A.N.; Tsitsilonis, S. -

The Biomechanics and Evolution of Impact Resistance in Human Walking and Running
The biomechanics and evolution of impact resistance in human walking and running The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Addison, Brian. 2016. The biomechanics and evolution of impact resistance in human walking and running. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:26718734 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA The biomechanics and evolution of impact resistance in human walking and running A dissertation presented by Brian James Addison to The Department of Human Evolutionary Biology in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Human Evolutionary Biology Harvard University Cambridge, Massachusetts November 2015 © 2016 Brian James Addison All rights reserved. Dissertation Adviser: Professor Daniel E. Lieberman Brian James Addison The biomechanics and evolution of impact resistance in human walking and running Abstract How do humans generate and resist repetitive impact forces beneath the heel during walking and heel strike running? Due to the evolution of long day ranges and larger body sizes in the hominin lineage modern human hunter-gatherers must resist millions of high magnitude impact forces per foot per year. As such, impact forces may have been a selective pressure on many aspects of human morphology, including skeletal structure. -

OITE Foot and Ankle Review Anatomy and Biomechanics
OITE Foot and Ankle Review Anatomy and Biomechanics • Bones and Ligaments – The Ankle Joint • Includes the Tibia, talus and fibula • Joint is trapezoidal and wider anteriorly • Talus only tarsal bone without muscular or ligamentous insertions – Lateral Ankle Ligaments • Anterior Talofibular Ligament (ATFL) – Under strain in plantar flexion, inversion, and Internal rotation • Calcaneofibular Ligament (CFL) – Under strain in dorsiflexion and inversion • Posterior Talofibular Ligament (PTFL) Anatomy and Biomechanics • Deltoid Ligament – Triangular shaped ligament with the apex at the medial malleolus and extending to the calcaneus, talus, and navicular – Divided into: • Superficial component – Three parts: anterior to navicular, inferior to sustanaculum, and posterior to talar body • Deep component – Two bands from medial malleolus to talar body just inferior to the medial facet • Syndesmosis – Ligamentous structures • Anterior inferior tibiofibular ligament (AITFL) • Interosseous ligament • Posterior inferior tibiofibular ligament • Transverse tibiofibular ligament Anatomy and Biomechanics • Hindfoot and Midfoot – Subtalar Joint • Three Facets: Posterior (Largest), Middle (medial and rests on sustenacum tali), and anterior (continuous with talonavicular joint) • Transverse Tarsal Joint (Chopart Joint) – Talonavicular and Calcaneocuboid Joints – Talonavicular Joint supported by two ligaments (Spring Ligament): the superior medial calcaneonavicular ligament (SMCN) and the inferior calcaneonavicular (ICN) ligament • Most likely attenuated in -

Bone Diagram.Pub
Bone Diagram Forehead The common name of (Frontal bone) each bone is listed first, Nose bones with the scientific name (Nasals) given in parenthesis. Cheek bone (Zygoma) Upper jaw (Maxilla) Lower jaw (Mandible) Collar bone (Clavicle) Breast bone (Sternum) Upper arm bone (Humerus) Lower arm bone (Ulna) Lower arm bone (Radius) Thigh bone (Femur) Kneecap (Patella) Shin bone (Tibia) Calf bone (Fibula) Ankle bones (Tarsals) Foot bones (Metatarsals) Toe bones (Phalanges) Skull Side of skull (Cranium) (Parietal bone) Back of skull Did you know? (Occipital bone) When you are a baby you Temple have more than 300 (Temporal bone) bones. By the time you are an adult you only Backbone Neck vertebrae (7) have 206 bones, because (Spine) (Cervical vertebrae) some of your bones join together as you grow! Shoulder blade (Scapula) Chest vertebrae (12) (Thoracic vertebrae) Ribs (12 pairs) Lower back vertebrae (5) (Lumbar vertebrae) Fused vertebrae (5) (Sacrum) Pelvic bones (Ilium) (Pubis) (Ischium) Wrist bones (Carpals) Hand bones (Metacarpals) Finger bones Bones are important! (Phalanges) They hold up your body, and along with your muscles, keep you moving. Without your bones, you’d just be one big blob! To be able to grow, strong bones needs lots of calcium and weight-bearing physical activity. Heel bone (Calcaneus) University of Washington PKU Clinic CHDD - Box 357920, Seattle, WA 98195 (206) 685-3015, Toll Free in Washington State 877-685-3015 http://depts.washington.edu/pku .