Examination of Shape Variation of the Calcaneus, Navicular, and Talus In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
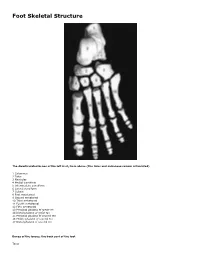
Skeletal Foot Structure
Foot Skeletal Structure The disarticulated bones of the left foot, from above (The talus and calcaneus remain articulated) 1 Calcaneus 2 Talus 3 Navicular 4 Medial cuneiform 5 Intermediate cuneiform 6 Lateral cuneiform 7 Cuboid 8 First metatarsal 9 Second metatarsal 10 Third metatarsal 11 Fourth metatarsal 12 Fifth metatarsal 13 Proximal phalanx of great toe 14 Distal phalanx of great toe 15 Proximal phalanx of second toe 16 Middle phalanx of second toe 17 Distal phalanx of second toe Bones of the tarsus, the back part of the foot Talus Calcaneus Navicular bone Cuboid bone Medial, intermediate and lateral cuneiform bones Bones of the metatarsus, the forepart of the foot First to fifth metatarsal bones (numbered from the medial side) Bones of the toes or digits Phalanges -- a proximal and a distal phalanx for the great toe; proximal, middle and distal phalanges for the second to fifth toes Sesamoid bones Two always present in the tendons of flexor hallucis brevis Origin and meaning of some terms associated with the foot Tibia: Latin for a flute or pipe; the shin bone has a fanciful resemblance to this wind instrument. Fibula: Latin for a pin or skewer; the long thin bone of the leg. Adjective fibular or peroneal, which is from the Greek for pin. Tarsus: Greek for a wicker frame; the basic framework for the back of the foot. Metatarsus: Greek for beyond the tarsus; the forepart of the foot. Talus (astragalus): Latin (Greek) for one of a set of dice; viewed from above the main part of the talus has a rather square appearance. -

Comparative Locomotor Performance of Marsupial and Placental Mammals
J. Zool., Lond. (1988) 215, 505-522 Comparative locomotor performance of marsupial and placental mammals T. GARLAND,JR Department of Zoology, University of Wisconsin, Madison, WZ 53706, USA School of Biological Sciences, The Flinders University of South Australia, Bedford Park, Adelaide, 5042, South Australia (Accepted 13 October 1987) (With 6 figures in the text) Marsupials are often considered inferior to placental mammals in a number of physiological characters. Because locomotor performance is presumed to be an important component of fitness, we compared marsupials and placentals with regard to both maximal running speeds and maximal aerobic speeds (=speed at which the maximal rate of oxygen consumption, \jozmax, is attained). Maximal aerobic speed is related to an animal's maximal sustainable speed, and hence is a useful comparative index of stamina. Maximal running speeds of 1 I species of Australian marsupials, eight species of Australian murid rodents, two species of American didelphid marsupials, and two species of American rodents were measured in the laboratory and compared with data compiled from the literature. Our values are greater than, or equivalent to, those reported previously. Marsupials and placentals do not differ in maximal running speeds (nor do Australian rodents differ from non- Australian rodents). Within these groups, however, species and families may differ considerably. Some of the interspecific variation in maximal running speeds is related to differences in habitat: species inhabiting open habitats (e.g. deserts) tend to be faster than are species from habitats with more cover, or arboreal species. Maximal aerobic speeds (compiled from the literature) were higher in large species than in small species. -

Rethinking the Evolution of the Human Foot: Insights from Experimental Research Nicholas B
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174425. doi:10.1242/jeb.174425 REVIEW Rethinking the evolution of the human foot: insights from experimental research Nicholas B. Holowka* and Daniel E. Lieberman* ABSTRACT presumably owing to their lack of arches and mobile midfoot joints Adaptive explanations for modern human foot anatomy have long for enhanced prehensility in arboreal locomotion (see Glossary; fascinated evolutionary biologists because of the dramatic differences Fig. 1B) (DeSilva, 2010; Elftman and Manter, 1935a). Other studies between our feet and those of our closest living relatives, the great have documented how great apes use their long toes, opposable apes. Morphological features, including hallucal opposability, toe halluces and mobile ankles for grasping arboreal supports (DeSilva, length and the longitudinal arch, have traditionally been used to 2009; Holowka et al., 2017a; Morton, 1924). These observations dichotomize human and great ape feet as being adapted for bipedal underlie what has become a consensus model of human foot walking and arboreal locomotion, respectively. However, recent evolution: that selection for bipedal walking came at the expense of biomechanical models of human foot function and experimental arboreal locomotor capabilities, resulting in a dichotomy between investigations of great ape locomotion have undermined this simple human and great ape foot anatomy and function. According to this dichotomy. Here, we review this research, focusing on the way of thinking, anatomical features of the foot characteristic of biomechanics of foot strike, push-off and elastic energy storage in great apes are assumed to represent adaptations for arboreal the foot, and show that humans and great apes share some behavior, and those unique to humans are assumed to be related underappreciated, surprising similarities in foot function, such as to bipedal walking. -

5Th Metatarsal Fracture
FIFTH METATARSAL FRACTURES Todd Gothelf MD (USA), FRACS, FAAOS, Dip. ABOS Foot, Ankle, Shoulder Surgeon Orthopaedic You have been diagnosed with a fracture of the fifth metatarsal bone. Surgeons This tyPe of fracture usually occurs when the ankle suddenly rolls inward. When the ankle rolls, a tendon that is attached to the fifth metatarsal bone is J. Goldberg stretched. Because the bone is weaker than the tendon, the bone cracks first. A. Turnbull R. Pattinson A. Loefler All bones heal in a different way when they break. This is esPecially true J. Negrine of the fifth metatarsal bone. In addition, the blood suPPly varies to different I. PoPoff areas, making it a lot harder for some fractures to heal without helP. Below are D. Sher descriPtions of the main Patterns of fractures of the fifth metatarsal fractures T. Gothelf and treatments for each. Sports Physicians FIFTH METATARSAL AVULSION FRACTURE J. Best This fracture Pattern occurs at the tiP of the bone (figure 1). These M. Cusi fractures have a very high rate of healing and require little Protection. Weight P. Annett on the foot is allowed as soon as the Patient is comfortable. While crutches may helP initially, walking without them is allowed. I Prefer to Place Patients in a walking boot, as it allows for more comfortable walking and Protects the foot from further injury. RICE treatment is initiated. Pain should be exPected to diminish over the first four weeks, but may not comPletely go away for several months. Follow-uP radiographs are not necessary if the Pain resolves as exPected. -

Conference Main Sponsors-2018
Anatomical variation of habitat related changes in scapular morphology C. Luziga1 and N. Wada2 1Department of Veterinary Anatomy and Pathology, College of Veterinary and Biomedical Sciences, Sokoine University of Agriculture, Morogoro, Tanzania 2Laboratory Physiology, Department of Veterinary Sciences, School of Veterinary Medicine, Yamaguchi University, Yamaguchi 753-8558, Japan E-mail: [email protected] SUMMARY The mammalian forelimb is adapted to different functions including postural, locomotor, feeding, exploratory, grooming and defense. Comparative studies on morphology of the mammalian scapula have been performed in an attempt to establish the functional differences in the use of the forelimb. In this study, a total of 102 scapulae collected from 66 species of animals, representatives of all major taxa from rodents, sirenians, marsupials, pilosa, cetaceans, carnivores, ungulates, primates and apes were analyzed. Parameters measured included scapular length, width, position, thickness, area, angles and index. Structures included supraspinous and infraspinous fossae, scapular spine, glenoid cavity, acromium and coracoid processes. Images were taken using computed tomographic (CT) scanning technology (CT-Aquarium, Toshiba and micro CT- LaTheta, Hotachi, Japan) and measurement values acquired and processed using Avizo computer software and CanvasTM 11 ACD systems. Statistical analysis was performed using Microsoft Excel 2013. Results obtained showed that there were similar morphological characteristics of scapula in mammals with arboreal locomotion and living in forest and mountainous areas but differed from those with leaping and terrestrial locomotion living in open habitat or savannah. The cause for the statistical grouping of the animals signifies presence of the close relationship between habitat and scapular morphology and in a way that corresponds to type of locomotion and speed. -

Orangutan Positional Behavior and the Nature of Arboreal Locomotion in Hominoidea Susannah K.S
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 000:000–000 (2006) Orangutan Positional Behavior and the Nature of Arboreal Locomotion in Hominoidea Susannah K.S. Thorpe1* and Robin H. Crompton2 1School of Biosciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK 2Department of Human Anatomy and Cell Biology, University of Liverpool, Liverpool L69 3GE, UK KEY WORDS Pongo pygmaeus; posture; orthograde clamber; forelimb suspend ABSTRACT The Asian apes, more than any other, are and orthograde compressive locomotor modes are ob- restricted to an arboreal habitat. They are consequently an served more frequently. Given the complexity of orangu- important model in the interpretation of the morphological tan positional behavior demonstrated by this study, it is commonalities of the apes, which are locomotor features likely that differences in positional behavior between associated with arboreal living. This paper presents a de- studies reflect differences in the interplay between the tailed analysis of orangutan positional behavior for all age- complex array of variables, which were shown to influence sex categories and during a complete range of behavioral orangutan positional behavior (Thorpe and Crompton [2005] contexts, following standardized positional mode descrip- Am. J. Phys. Anthropol. 127:58–78). With the exception tions proposed by Hunt et al. ([1996] Primates 37:363–387). of pronograde suspensory posture and locomotion, orang- This paper shows that orangutan positional behavior is utan positional behavior is similar to that of the African highly complex, representing a diverse spectrum of posi- apes, and in particular, lowland gorillas. This study sug- tional modes. Overall, all orthograde and pronograde sus- gests that it is orthogrady in general, rather than fore- pensory postures are exhibited less frequently in the pres- limb suspend specifically, that characterizes the posi- ent study than previously reported. -

The Biodynamics of Arboreal Locomotion in the Gray Short
THE BIODYNAMICS OF ARBOREAL LOCOMOTION IN THE GRAY SHORT- TAILED OPOSSUM (MONODELPHIS DOMESTICA) A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Andrew R. Lammers August 2004 This dissertation entitled THE BIODYNAMICS OF ARBOREAL LOCOMOTION IN THE GRAY SHORT- TAILED OPOSSUM (MONODELPHIS DOMESTICA) BY ANDREW R. LAMMERS has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Audrone R. Biknevicius Associate Professor of Biomedical Sciences Leslie A. Flemming Dean, College of Arts and Sciences LAMMERS, ANDREW R. Ph.D. August 2004. Biological Sciences The biodynamics of arboreal locomotion in the gray short-tailed opossum (Monodelphis domestica). (147 pp.) Director of Dissertation: Audrone R. Biknevicius Most studies of animal locomotor biomechanics examine movement on a level, flat trackway. However, small animals must negotiate heterogenerous terrain that includes changes in orientation and diameter. Furthermore, animals which are specialized for arboreal locomotion may solve the biomechanical problems that are inherent in substrates that are sloped and/or narrow differently from animals which are considered terrestrial. Thus I studied the effects of substrate orientation and diameter on locomotor kinetics and kinematics in the gray short-tailed opossum (Monodelphis domestica). The genus Monodelphis is considered the most terrestrially adapted member of the family Didelphidae, but nevertheless these opossums are reasonably skilled at climbing. The first study (Chapter 2) examines the biomechanics of moving up a 30° incline and down a 30° decline. Substrate reaction forces (SRFs), limb kinematics, and required coefficient of friction were measured. -

Hand Pressures During Arboreal Locomotion in Captive Bonobos (Pan Paniscus) Diana S
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb170910. doi:10.1242/jeb.170910 RESEARCH ARTICLE Hand pressures during arboreal locomotion in captive bonobos (Pan paniscus) Diana S. Samuel1, Sandra Nauwelaerts2,3, Jeroen M. G. Stevens3,4 and Tracy L. Kivell1,5,* ABSTRACT of both manipulation and locomotion. Although there has been much Evolution of the human hand has undergone a transition from use research into the potential changes in manipulative abilities during locomotion to use primarily for manipulation. Previous throughout human evolution, from both morphological (e.g. comparative morphological and biomechanical studies have Napier, 1955; Marzke, 1997; Marzke et al., 1999; Skinner et al., focused on potential changes in manipulative abilities during 2015) and biomechanical (e.g. Marzke et al., 1998; Rolian et al., human hand evolution, but few have focused on functional signals 2011; Williams et al., 2012; Key and Dunmore, 2014) perspectives, for arboreal locomotion. Here, we provide this comparative context comparatively little research has been done that may help us infer though the first analysis of hand loading in captive bonobos during how our ancestors may have used their hands for arboreal arboreal locomotion. We quantify pressure experienced by the locomotion, particularly climbing and suspension. Many fossil fingers, palm and thumb in bonobos during vertical locomotion, hominins show features of the hand (e.g. curved fingers) and upper suspension and arboreal knuckle-walking. The results show that limb (e.g. superiorly oriented shoulder joint) (e.g. Stern, 2000; pressure experienced by the fingers is significantly higher during Larson, 2007; Churchill et al., 2013; Kivell et al., 2011, 2015; Kivell, knuckle-walking compared with similar pressures experienced by the 2015) that suggest arboreal locomotion may still have been an fingers and palm during suspensory and vertical locomotion. -

Analysis of the Talus and Calcaneus Bones from the Poole-Rose Ossuary
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2005 Analysis of the talus and calcaneus bones from the Poole-Rose Ossuary: a Late Woodland burial site in Ontario, Canada Adrienne Elizabeth Penney Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Social and Behavioral Sciences Commons Recommended Citation Penney, Adrienne Elizabeth, "Analysis of the talus and calcaneus bones from the Poole-Rose Ossuary: a Late Woodland burial site in Ontario, Canada" (2005). LSU Master's Theses. 3114. https://digitalcommons.lsu.edu/gradschool_theses/3114 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. ANALYSIS OF THE TALUS AND CALCANEUS BONES FROM THE POOLE-ROSE OSSUARY: A LATE WOODLAND BURIAL SITE IN ONTARIO, CANADA A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Arts in The Department of Geography and Anthropology by Adrienne Elizabeth Penney B.A. University of Evansville, 2003 August 2005 ACKNOWLEDGEMENTS I appreciate the cooperation of the Alderville First Nation, and especially Nora Bothwell, for allowing the study of the valuable Poole-Rose collection. I would like to thank Ms. Mary H. Manhein, my advisor, for all of her time, encouragement, and assistance. -

Management of Intra-Articular Fractures of the Calcaneus: Introducing a New Locking Plate
Shafa Ortho J. 2018 November; 5(4):e7991. doi: 10.5812/soj.7991. Published online 2018 October 9. Letter Management of Intra-Articular Fractures of the Calcaneus: Introducing a New Locking Plate Bijan Valiollahi 1, * and Mostafa Salariyeh 1 1Bone and Joint Reconstruction Research Center, Shafa Orthopedic Hospital, Iran University of Medical Sciences, Tehran, Iran *Corresponding author: Bone and Joint Reconstruction Research Center, Shafa Orthopedic Hospital, Iran University of Medical Sciences, Tehran, Iran. Email: [email protected] Received 2016 October 31; Revised 2018 September 22; Accepted 2018 September 27. Keywords: Calcaneus Fracture, New Plate, Sinus Tarsal Approach Dear Editor, displaced intra-articular fractures involving the posterior Fracture of the calcaneus is usually a challenging prob- facet and is ideally performed within three weeks of in- lem for orthopedic surgeons and patients. Calcaneal frac- jury. Beyond this time, separation of the fragments be- tures are the most common of tarsal bone fractures, and comes more challenging. To achieve an adequate reduc- most of them are displaced intra-articular (nearly 75%) frac- tion, surgery must be performed after soft tissue swelling tures, usually resulting from falling or motor vehicle acci- diminishes (2). dents (1). Full diminishing of soft tissue edema is demonstrated Most patients with calcaneal fractures are usually by a positive wrinkle test, indicating that surgical interven- young men in their prime working years, which results in tion may be performed safely. For the extensile lateral ap- a significant loss of economic productivity. Although sur- proach, a good skin with positive wrinkle test is needed. gical techniques and fixation implants have generally im- Concerns about high wound complication rates in the ex- proved functional outcomes, there is vast controversy as to tensile lateral approach have prompted some to improve the management of these highly complex injuries. -

Autogenous Calcaneal Dowel Grafting Jones Fractures: Technique and Case Study Edward G
Autogenous Calcaneal Dowel Grafting Jones Fractures: Technique and Case Study Edward G. Blahous JR, DPM, FACFAS, Richard T. Bouché, DPM, FACFAS, Amol Saxena, DPM, FACFAS, FAAPSM, Chad Seidenstricker, DPM, PGY-3 A trephine is now used to obtain a bone graft plug, which will be used as an inlay graft to be placed into reamed hole. To assure a tight custom fit trephine diameter should be Introduction 1 mm greater than the reamer size. The authors’ prefer use of bone graft procured Many foot and ankle surgeons elect to treat the proximal fifth metatarsal base from the lateral calcaneal wall (Fig 6). The bone plug graft is then gently aligned and fractures near the metaphyseal-diaphyseal junction by surgical repair due to tamped into place (Fig 8). unpredictable outcomes with nonoperative management. Intramedullary fixation (IM) is the current “gold standard” and has high union rate [1]. However, there are several reports of complications with the IM technique. Notably, refracture, prominent hardware, and slow time to fracture healing [2-5]. This publication aims to describe a novel technique to treat the problematic Jones fracture. Figure 10. Intraoperative anteroposterior fluoroscopic Figure 11. Intraoperative lateral fluoroscopic view Figure 6. Incision placement with trephine in Figure 5. Visualization of recipient hole view of fracture site with graft and fixation in place. of fracture site with graft and fixation in place. Surgical Technique [6] place for safe exposure of lateral calcaneal through fracture site. The patient is placed in a lateral decubitus position with ipsilateral side facing wall. superiorly. A dorsolateral incision is made centered over the fifth metatarsal base Discussion fracture site. -

The Biodynamics of Arboreal Locomotion: the Effects of Substrate Diameter on Locomotor Kinetics in the Gray Short-Tailed Opossum (Monodelphis Domestica) Andrew R
The Journal of Experimental Biology 207, 4325-4336 4325 Published by The Company of Biologists 2004 doi:10.1242/jeb.01231 The biodynamics of arboreal locomotion: the effects of substrate diameter on locomotor kinetics in the gray short-tailed opossum (Monodelphis domestica) Andrew R. Lammers1,* and Audrone R. Biknevicius2 1Department of Biological Sciences, Ohio University, Athens, OH 45701, USA and 2Department of Biomedical Sciences, Ohio University College of Osteopathic Medicine, Athens, OH 45701, USA *Author for correspondence at present address: Department of Health Sciences, Cleveland State University, Cleveland, OH 44115, USA (e-mail: [email protected]) Accepted 9 August 2004 Summary Effects of substrate diameter on locomotor biodynamics segregated between limbs on the terrestrial trackway, fore were studied in the gray short-tailed opossum limbs were dominant both in braking and in propulsion on (Monodelphis domestica). Two horizontal substrates were the arboreal trackway. Both fore and hind limbs exerted used: a flat ‘terrestrial’ trackway with a force platform equivalently strong, medially directed limb forces on the integrated into the surface and a cylindrical ‘arboreal’ arboreal trackway and laterally directed limb forces on the trackway (20.3·mm diameter) with a force-transducer terrestrial trackway. We propose that the modifications in instrumented region. On both terrestrial and arboreal substrate reaction force on the arboreal trackway are due substrates, fore limbs exhibited higher vertical impulse and to the differential placement of the limbs about the peak vertical force than hind limbs. Although vertical limb dorsolateral aspect of the branch. Specifically, the pes impulses were lower on the terrestrial substrate than on typically made contact with the branch lower and more the arboreal support, this was probably due to speed laterally than the manus, which may explain the effects because the opossums refused to move as quickly on significantly lower required coefficient of friction in the the arboreal trackway.