Rethinking the Evolution of the Human Foot: Insights from Experimental Research Nicholas B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
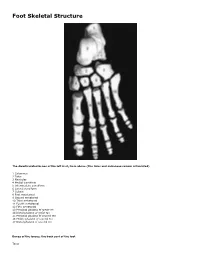
Skeletal Foot Structure
Foot Skeletal Structure The disarticulated bones of the left foot, from above (The talus and calcaneus remain articulated) 1 Calcaneus 2 Talus 3 Navicular 4 Medial cuneiform 5 Intermediate cuneiform 6 Lateral cuneiform 7 Cuboid 8 First metatarsal 9 Second metatarsal 10 Third metatarsal 11 Fourth metatarsal 12 Fifth metatarsal 13 Proximal phalanx of great toe 14 Distal phalanx of great toe 15 Proximal phalanx of second toe 16 Middle phalanx of second toe 17 Distal phalanx of second toe Bones of the tarsus, the back part of the foot Talus Calcaneus Navicular bone Cuboid bone Medial, intermediate and lateral cuneiform bones Bones of the metatarsus, the forepart of the foot First to fifth metatarsal bones (numbered from the medial side) Bones of the toes or digits Phalanges -- a proximal and a distal phalanx for the great toe; proximal, middle and distal phalanges for the second to fifth toes Sesamoid bones Two always present in the tendons of flexor hallucis brevis Origin and meaning of some terms associated with the foot Tibia: Latin for a flute or pipe; the shin bone has a fanciful resemblance to this wind instrument. Fibula: Latin for a pin or skewer; the long thin bone of the leg. Adjective fibular or peroneal, which is from the Greek for pin. Tarsus: Greek for a wicker frame; the basic framework for the back of the foot. Metatarsus: Greek for beyond the tarsus; the forepart of the foot. Talus (astragalus): Latin (Greek) for one of a set of dice; viewed from above the main part of the talus has a rather square appearance. -

Sole Training® with Stacey Lei Krauss
Sole Training® with Stacey Lei Krauss Sole Training is a foot fitness program based on two sequences. The self –massage sequence is restorative and therapeutic; compare it to a yoga class (for your feet). The standing sequence promotes strength, endurance, flexibility and coordination; compare it to a boot-camp workout (for your feet). These exercises work; we’ve been doing them for over a decade. *The Sole Training® video download is available at willPowerMethod.com What is foot fitness? Building muscular strength, endurance, flexibility and neuro-muscular awareness in the feet and ankles. What are the benefits of foot fitness? According to Vibram FiveFingers®, exercising while barefoot, or wearing minimal shoes provide the following benefits: 1. Strengthens Muscles in the Feet and Lower Legs Wearing minimal shoes, or training barefoot will stimulate and strengthen muscles in the feet and lower legs, improving general foot health and reducing the risk of injury. 2. Improves Range of Motion in Ankles, Feet and Toes No longer 'cast' in a traditional, structured shoe, the foot and toes move more naturally. 3. Stimulates Neural Function Important to Balance and Agility When barefoot or wearing minimal shoes, thousands of neurological receptors in the feet send valuable information to the brain, improving balance and agility. 4. Eliminate Heel Lift to Align the Spine and Improve Posture By lowering the heel, your bodyweight becomes evenly distributed across the footbed, promoting proper posture and spinal alignment. 5. Allow the Foot and Body to Move Naturally Which just FEELS GOOD. [email protected] Sole Training® 1 Sole Training® with Stacey Lei Krauss Sole Training® Massage Sequence preparation: mats, blankets, blocks, towels, foot lotion time: 3-10 minutes when: prior to any workout, after any workout, before bed or upon waking EXERCISE EXECUTION FUNCTION Use your fingers to lengthen your toes: LOCALLY: Circulation, Toe flexibility and mobility leading TOE • Long stretch (3 joints except Big Toe) to enhanced balance. -

Foot and Ankle Motion Analysis Using Dynamic Radiographic Imaging Benjamin Donald Mchenry Marquette University
Marquette University e-Publications@Marquette Dissertations (2009 -) Dissertations, Theses, and Professional Projects Foot and Ankle Motion Analysis Using Dynamic Radiographic Imaging Benjamin Donald McHenry Marquette University Recommended Citation McHenry, Benjamin Donald, "Foot and Ankle Motion Analysis Using Dynamic Radiographic Imaging" (2013). Dissertations (2009 -). Paper 276. http://epublications.marquette.edu/dissertations_mu/276 FOOT AND ANKLE MOTION ANALYSIS USING DYNAMIC RADIOGRAPHIC IMAGING by Benjamin D. McHenry, B.S. A Dissertation submitted to the Faculty of the Graduate School, Marquette University, in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Milwaukee, Wisconsin May 2013 ABSTRACT FOOT AND ANKLE MOTION ANALYSIS USING DYNAMIC RADIOGRAPHIC IMAGING Benjamin D. McHenry, B.S. Marquette University, 2013 Lower extremity motion analysis has become a powerful tool used to assess the dynamics of both normal and pathologic gait in a variety of clinical and research settings. Early rigid representations of the foot have recently been replaced with multi-segmental models capable of estimating intra-foot motion. Current models using externally placed markers on the surface of the skin are easily implemented, but suffer from errors associated with soft tissue artifact, marker placement repeatability, and rigid segment assumptions. Models using intra-cortical bone pins circumvent these errors, but their invasive nature has limited their application to research only. Radiographic models reporting gait kinematics currently analyze progressive static foot positions and do not include dynamics. The goal of this study was to determine the feasibility of using fluoroscopy to measure in vivo intra-foot dynamics of the hindfoot during the stance phase of gait. The developed fluoroscopic system was synchronized to a standard motion analysis system which included a multi-axis force platform. -

Comparative Locomotor Performance of Marsupial and Placental Mammals
J. Zool., Lond. (1988) 215, 505-522 Comparative locomotor performance of marsupial and placental mammals T. GARLAND,JR Department of Zoology, University of Wisconsin, Madison, WZ 53706, USA School of Biological Sciences, The Flinders University of South Australia, Bedford Park, Adelaide, 5042, South Australia (Accepted 13 October 1987) (With 6 figures in the text) Marsupials are often considered inferior to placental mammals in a number of physiological characters. Because locomotor performance is presumed to be an important component of fitness, we compared marsupials and placentals with regard to both maximal running speeds and maximal aerobic speeds (=speed at which the maximal rate of oxygen consumption, \jozmax, is attained). Maximal aerobic speed is related to an animal's maximal sustainable speed, and hence is a useful comparative index of stamina. Maximal running speeds of 1 I species of Australian marsupials, eight species of Australian murid rodents, two species of American didelphid marsupials, and two species of American rodents were measured in the laboratory and compared with data compiled from the literature. Our values are greater than, or equivalent to, those reported previously. Marsupials and placentals do not differ in maximal running speeds (nor do Australian rodents differ from non- Australian rodents). Within these groups, however, species and families may differ considerably. Some of the interspecific variation in maximal running speeds is related to differences in habitat: species inhabiting open habitats (e.g. deserts) tend to be faster than are species from habitats with more cover, or arboreal species. Maximal aerobic speeds (compiled from the literature) were higher in large species than in small species. -

Locomotor Training with Partial Body Weight Support in Spinal Cord Injury Rehabilitation: Literature Review
doi: ISSN 0103-5150 Fisioter. Mov., Curitiba, v. 26, n. 4, p.página 907-920, set./dez. 2013 Licenciado sob uma Licença Creative Commons [T] Treino locomotor com suporte parcial de peso corporal na reabilitação da lesão medular: revisão da literatura [I] Locomotor training with partial body weight support in spinal cord injury rehabilitation: literature review [A] Cristina Maria Rocha Dutra[a], Cynthia Maria Rocha Dutra[b], Auristela Duarte de Lima Moser[c], Elisangela Ferretti Manffra[c] [a] Mestranda do Programa de Pós-Graduação em Tecnologia em Saúde da Pontiícia Universidade Católica do Paraná (PUCPR), Curitiba, PR - Brasil, e-mail: [email protected] [b] Professora mestre da Universidade Tuiuti do PR - Curitiba, Paraná, Brasil, e-mail: [email protected] [c] Professoras doutoras do Programa de Pós-Graduação em Tecnologia em Saúde da Pontiícia Universidade Católica do Paraná, Curitiba, P - Brasil, e-mails: [email protected], [email protected] [R] Resumo Introdução: O treino locomotor com suporte de peso corporal (TLSP) é utilizado há aproximadamente 20 anos no campo da reabilitação em pacientes que sofrem de patologias neurológicas. O TLSP favorece melhoras osteomusculares, cardiovasculares e psicológicas, pois desenvolve ao máximo o potencial residual do orga- nismo, proporcionando a reintegração na convivência familiar, proissional e social. Objetivo: Identiicar as principais modalidades de TLSP e seus parâmetros de avaliação com a inalidade de contribuir com o esta- belecimento de evidências coniáveis para as práticas reabilitativas de pessoas com lesão medular. Materiais e métodos: Foram analisados artigos originais, publicados entre 2000 e 2011, que envolvessem treino de marcha após a lesão medular, com ou sem suporte parcial de peso corporal, e tecnologias na assistência do treino, como biofeedback e estimulação elétrica funcional, entre outras. -

The Evolution of Micro-Cursoriality in Mammals
© 2014. Published by The Company of Biologists Ltd | The Journal of Experimental Biology (2014) 217, 1316-1325 doi:10.1242/jeb.095737 RESEARCH ARTICLE The evolution of micro-cursoriality in mammals Barry G. Lovegrove* and Metobor O. Mowoe* ABSTRACT Perissodactyla) in response to the emergence of open landscapes and In this study we report on the evolution of micro-cursoriality, a unique grasslands following the Eocene Thermal Maximum (Janis, 1993; case of cursoriality in mammals smaller than 1 kg. We obtained new Janis and Wilhelm, 1993; Yuanqing et al., 2007; Jardine et al., 2012; running speed and limb morphology data for two species of elephant- Lovegrove, 2012b; Lovegrove and Mowoe, 2013). shrews (Elephantulus spp., Macroscelidae) from Namaqualand, Loosely defined, cursorial mammals are those that run fast. South Africa, which we compared with published data for other However, more explicit definitions of cursoriality remain obscure mammals. Elephantulus maximum running speeds were higher than because locomotor performance is influenced by multiple variables, those of most mammals smaller than 1 kg. Elephantulus also including behaviour, biomechanics, physiology and morphology possess exceptionally high metatarsal:femur ratios (1.07) that are (Taylor et al., 1970; Garland, 1983a; Garland, 1983b; Garland and typically associated with fast unguligrade cursors. Cursoriality evolved Janis, 1993; Stein and Casinos, 1997; Carrano, 1999). In an in the Artiodactyla, Perissodactyla and Carnivora coincident with evaluation of these definition problems, Carrano (Carrano, 1999) global cooling and the replacement of forests with open landscapes argued that ‘…morphology should remain the fundamental basis for in the Oligocene and Miocene. The majority of mammal species, making distinctions between locomotor performance…’. -

Multi-Segment Foot Models and Their Use in Clinical Populations
Gait & Posture 69 (2019) 50–59 Contents lists available at ScienceDirect Gait & Posture journal homepage: www.elsevier.com/locate/gaitpost Review Multi-segment foot models and their use in clinical populations T ⁎ Alberto Leardinia,1, Paolo Caravaggia, ,1, Tim Theologisb,2, Julie Stebbinsb,2 a Movement Analysis Laboratory, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy b Oxford Gait Laboratory, Nuffield Orthopaedic Centre, Oxford, UK ARTICLE INFO ABSTRACT Keywords: Background: Many multi-segment foot models based on skin-markers have been proposed for in-vivo kinematic Foot joints analysis of foot joints. It remains unclear whether these models have developed far enough to be useful in clinical Kinematics populations. The present paper aims at reviewing these models, by discussing major methodological issues, and Multisegment foot models analyzing relevant clinical applications. Clinical gait analysis Research question: Can multi-segment foot models be used in clinical populations? Foot pathologies Methods: Pubmed and Google Scholar were used as the main search engines to perform an extensive literature search of papers reporting definition, validation or application studies of multi-segment foot models. The search keywords were the following: ‘multisegment’; ‘foot’; ‘model’; ‘kinematics’, ‘joints’ and ‘gait’. Results: More than 100 papers published between 1991 and 2018 were identified and included in the review. These studies either described a technique or reported a clinical application of one of nearly 40 models which differed according to the number of segments, bony landmarks, marker set, definition of anatomical frames, and convention for calculation of joint rotations. Only a few of these models have undergone robust validation studies. Clinical application papers divided by type of assessment revealed that the large majority of studies were a cross-sectional comparison of a pathological group to a control population. -

Multiplanar CT Analysis of Fifth Metatarsal Morphology
FAIXXX10.1177/1071100715623041Foot & Ankle InternationalDeSandis et al 623041research-article2015 Article Foot & Ankle International® 2016, Vol. 37(5) 528 –536 Multiplanar CT Analysis of Fifth Metatarsal © The Author(s) 2015 Reprints and permissions: sagepub.com/journalsPermissions.nav Morphology: Implications for Operative DOI: 10.1177/1071100715623041 Management of Zone II Fractures fai.sagepub.com Bridget DeSandis, BA1, Conor Murphy, MD2, Andrew Rosenbaum, MD1, Matthew Levitsky, BA1, Quinn O’Malley1, Gabrielle Konin, MD1, and Mark Drakos, MD1 Abstract Background: Percutaneous internal fixation is currently the method of choice treating proximal zone II fifth metatarsal fractures. Complications have been reported due to poor screw placement and inadequate screw sizing. The purpose of this study was to define the morphology of the fifth metatarsal to help guide surgeons in selecting the appropriate screw size preoperatively. Methods: Multiplanar analysis of fifth metatarsal morphology was completed using computed tomographic (CT) scans from 241 patients. Specific parameters were analyzed and defined in anteroposterior (AP), lateral, and oblique views including metatarsal length, distance from the base to apex of curvature, apex medullary canal width, apex height, and fifth metatarsal angle. Results: The average metatarsal length in the AP view was 71.4 ± 6.1 mm and in the lateral view 70.4 ± 6.0 mm, with 95% of patients having lengths between 59.3 and 83.5 mm and 58.4 and 82.4 mm, respectively. The average canal width at the apex of curvature was 4.1 ± 0.9 mm in the AP view and 5.3 ± 1.1 mm in the lateral view, with 95% of patients having widths between 2.2 and 5.9 mm and 3.2 and 7.5 mm, respectively. -

Gait Analysis in Uner Tan Syndrome Cases with Key Symptoms of Quadrupedal Locomotion, Mental Impairment, and Dysarthric Or No Speech
Article ID: WMC005017 ISSN 2046-1690 Gait Analysis in Uner Tan Syndrome Cases with Key Symptoms of Quadrupedal Locomotion, Mental Impairment, and Dysarthric or No Speech Peer review status: No Corresponding Author: Submitting Author: Prof. Uner Tan, Senior Researcher, Cukurova University Medical School , Cukurova University, Medical School, Adana, 01330 - Turkey Article ID: WMC005017 Article Type: Research articles Submitted on: 09-Nov-2015, 01:39:27 PM GMT Published on: 10-Nov-2015, 08:43:08 AM GMT Article URL: http://www.webmedcentral.com/article_view/5017 Subject Categories: NEUROSCIENCES Keywords: Uner Tan syndrome, quadrupedal locomotion, ataxia, gait, lateral sequence, diagonal sequence, evolution, primates How to cite the article: Tan U. Gait Analysis in Uner Tan Syndrome Cases with Key Symptoms of Quadrupedal Locomotion, Mental Impairment, and Dysarthric or No Speech. WebmedCentral NEUROSCIENCES 2015;6(11):WMC005017 Copyright: This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Source(s) of Funding: None Competing Interests: None Additional Files: Illustration 1 ILLUSTRATION 2 ILLUSTRATION 3 ILLUSTRATION 4 ILLUSTRATION 5 ILLUSTRATION 6 WebmedCentral > Research articles Page 1 of 14 WMC005017 Downloaded from http://www.webmedcentral.com on 10-Nov-2015, 08:43:10 AM Gait Analysis in Uner Tan Syndrome Cases with Key Symptoms of Quadrupedal Locomotion, Mental Impairment, and Dysarthric or No Speech Author(s): Tan U Abstract sequence, on the lateral and on the diagonals.” This child typically exhibited straight legs during quadrupedal standing. Introduction: Uner Tan syndrome (UTS) consists of A man with healthy legs walking on all fours was quadrupedal locomotion (QL), impaired intelligence discovered by Childs [3] in Turkey, 1917. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

5Th Metatarsal Fracture
FIFTH METATARSAL FRACTURES Todd Gothelf MD (USA), FRACS, FAAOS, Dip. ABOS Foot, Ankle, Shoulder Surgeon Orthopaedic You have been diagnosed with a fracture of the fifth metatarsal bone. Surgeons This tyPe of fracture usually occurs when the ankle suddenly rolls inward. When the ankle rolls, a tendon that is attached to the fifth metatarsal bone is J. Goldberg stretched. Because the bone is weaker than the tendon, the bone cracks first. A. Turnbull R. Pattinson A. Loefler All bones heal in a different way when they break. This is esPecially true J. Negrine of the fifth metatarsal bone. In addition, the blood suPPly varies to different I. PoPoff areas, making it a lot harder for some fractures to heal without helP. Below are D. Sher descriPtions of the main Patterns of fractures of the fifth metatarsal fractures T. Gothelf and treatments for each. Sports Physicians FIFTH METATARSAL AVULSION FRACTURE J. Best This fracture Pattern occurs at the tiP of the bone (figure 1). These M. Cusi fractures have a very high rate of healing and require little Protection. Weight P. Annett on the foot is allowed as soon as the Patient is comfortable. While crutches may helP initially, walking without them is allowed. I Prefer to Place Patients in a walking boot, as it allows for more comfortable walking and Protects the foot from further injury. RICE treatment is initiated. Pain should be exPected to diminish over the first four weeks, but may not comPletely go away for several months. Follow-uP radiographs are not necessary if the Pain resolves as exPected. -

Common Stress Fractures BRENT W
COVER ARTICLE PRACTICAL THERAPEUTICS Common Stress Fractures BRENT W. SANDERLIN, LCDR, MC, USNR, Naval Branch Medical Clinic, Fort Worth, Texas ROBERT F. RASPA, CAPT, MC, USN, Naval Hospital Jacksonville, Jacksonville, Florida Lower extremity stress fractures are common injuries most often associated with partic- ipation in sports involving running, jumping, or repetitive stress. The initial diagnosis can be made by identifying localized bone pain that increases with weight bearing or repet- itive use. Plain film radiographs are frequently unrevealing. Confirmation of a stress frac- ture is best made using triple phase nuclear medicine bone scan or magnetic resonance imaging. Prevention of stress fractures is most effectively accomplished by increasing the level of exercise slowly, adequately warming up and stretching before exercise, and using cushioned insoles and appropriate footwear. Treatment involves rest of the injured bone, followed by a gradual return to the sport once free of pain. Recent evidence sup- ports the use of air splinting to reduce pain and decrease the time until return to full par- ticipation or intensity of exercise. (Am Fam Physician 2003;68:1527-32. Copyright© 2003 American Academy of Family Physicians) tress fractures are among the involving repetitive use of the arms, such most common sports injuries as baseball or tennis. Stress fractures of and are frequently managed the ribs occur in sports such as rowing. by family physicians. A stress Upper extremity and rib stress fractures fracture should be suspected in are far less common than lower extremity Sany patient presenting with localized stress fractures.1 bone or periosteal pain, especially if he or she recently started an exercise program Etiology and Pathophysiology or increased the intensity of exercise.