IR-FEL Experiments on Molecules and Clusters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Famous Physicists Himansu Sekhar Fatesingh
Fun Quiz FAMOUS PHYSICISTS HIMANSU SEKHAR FATESINGH 1. The first woman to 6. He first succeeded in receive the Nobel Prize in producing the nuclear physics was chain reaction. a. Maria G. Mayer a. Otto Hahn b. Irene Curie b. Fritz Strassmann c. Marie Curie c. Robert Oppenheimer d. Lise Meitner d. Enrico Fermi 2. Who first suggested electron 7. The credit for discovering shells around the nucleus? electron microscope is often a. Ernest Rutherford attributed to b. Neils Bohr a. H. Germer c. Erwin Schrödinger b. Ernst Ruska d. Wolfgang Pauli c. George P. Thomson d. Clinton J. Davisson 8. The wave theory of light was 3. He first measured negative first proposed by charge on an electron. a. Christiaan Huygens a. J. J. Thomson b. Isaac Newton b. Clinton Davisson c. Hermann Helmholtz c. Louis de Broglie d. Augustin Fresnel d. Robert A. Millikan 9. He was the first scientist 4. The existence of quarks was to find proof of Einstein’s first suggested by theory of relativity a. Max Planck a. Edwin Hubble b. Sheldon Glasgow b. George Gamow c. Murray Gell-Mann c. S. Chandrasekhar d. Albert Einstein d. Arthur Eddington 10. The credit for development of the cyclotron 5. The phenomenon of goes to: superconductivity was a. Carl Anderson b. Donald Glaser discovered by c. Ernest O. Lawrence d. Charles Wilson a. Heike Kamerlingh Onnes b. Alex Muller c. Brian D. Josephson 11. Who first proposed the use of absolute scale d. John Bardeen of Temperature? a. Anders Celsius b. Lord Kelvin c. Rudolf Clausius d. -

Ernest Rutherford and the Accelerator: “A Million Volts in a Soapbox”
Ernest Rutherford and the Accelerator: “A Million Volts in a Soapbox” AAPT 2011 Winter Meeting Jacksonville, FL January 10, 2011 H. Frederick Dylla American Institute of Physics Steven T. Corneliussen Jefferson Lab Outline • Rutherford's call for inventing accelerators ("million volts in a soap box") • Newton, Franklin and Jefferson: Notable prefiguring of Rutherford's call • Rutherfords's discovery: The atomic nucleus and a new experimental method (scattering) • A century of particle accelerators AAPT Winter Meeting January 10, 2011 Rutherford’s call for inventing accelerators 1911 – Rutherford discovered the atom’s nucleus • Revolutionized study of the submicroscopic realm • Established method of making inferences from particle scattering 1927 – Anniversary Address of the President of the Royal Society • Expressed a long-standing “ambition to have available for study a copious supply of atoms and electrons which have an individual energy far transcending that of the alpha and beta particles” available from natural sources so as to “open up an extraordinarily interesting field of investigation.” AAPT Winter Meeting January 10, 2011 Rutherford’s wish: “A million volts in a soapbox” Spurred the invention of the particle accelerator, leading to: • Rich fundamental understanding of matter • Rich understanding of astrophysical phenomena • Extraordinary range of particle-accelerator technologies and applications AAPT Winter Meeting January 10, 2011 From Newton, Jefferson & Franklin to Rutherford’s call for inventing accelerators Isaac Newton, 1717, foreseeing something like quarks and the nuclear strong force: “There are agents in Nature able to make the particles of bodies stick together by very strong attractions. And it is the business of Experimental Philosophy to find them out. -

Twenty Five Hundred Years of Small Science What’S Next?
Twenty Five Hundred Years of Small Science What’s Next? Lloyd Whitman Assistant Director for Nanotechnology White House Office of Science and Technology Policy Workshop on Integrated Nanosystems for Atomically Precise Manufacturing Berkeley, CA, August 5, 2015 Democritus (ca. 460 – 370 BC) Everything is composed of “atoms” Atomos (ἄτομος): that which can not be cut www.phil-fak.uni- duesseldorf.de/philo/galerie/antike/ demokrit.html Quantum Mechanics (1920s) Max Planck 1918* Albert Einstein 1921 Niels Bohr 1922 Louis de Broglie 1929 Max Born 1954 Paul Dirac 1933 On the Theory of Quanta Louis-Victor de Broglie Werner Heisenberg 1932 Wolfgang Pauli 1945 Erwin Schrödinger 1933 *Nobel Prizes in Physics https://tel.archives-ouvertes.fr/tel- 00006807 Ernst Ruska (1906 – 1988) Electron Microscopy Magnifying higher than the light microscope - 1933 Nobel Prize in Physics 1986 www.nobelprize.org/nobel_prizes/physics/laureates /1986/ruska-lecture.pdf Richard Feynman (1918-1988) There's Plenty of Room at the Bottom, An Invitation to Enter a New Field of Physics What would happen if we could arrange the atoms one by one the way we want them…? December 29, 1959 richard-feynman.net Heinrich Rohrer (1933 – 2013) Gerd Binnig Atomic resolution Scanning Tunneling Microscopy - 1981 1983 I could not stop looking at the images. It was like entering a new world. Gerd Binnig, Nobel lecture Binnig, et al., PRL 50, 120 (1983) Nobel Prize in Physics 1986 C60: Buckminsterfullerene Kroto, Heath, O‘Brien, Curl and September 1985 Smalley - 1985 …a remarkably stable cluster consisting of 60 carbon atoms…a truncated icosahedron. Nature 318, 162 (1985) http://www.acs.org/content/acs/en/education/whatis chemistry/landmarks/fullerenes.html Nobel Prize in Chemistry 1996 Curl, Kroto, and Smalley Positioning Single Atoms with a Scanning Tunnelling Microscope Eigler and Schweizer - 1990 …fabricate rudimentary structures of our own design, atom by atom. -

ERNST RUSKA Max-Eyth-Strasse 20, D-1000 BERLIN 33
THE DEVELOPMENT OF THE ELECTRON MICROSCOPE AND OF ELECTRON MICROSCOPY Nobel lecture, December 8, 1986 by ERNST RUSKA Max-Eyth-Strasse 20, D-1000 BERLIN 33 A. Parents’ house, family A month ago, the Nobel Foundation sent me its yearbook of 1985. From it I learnt that many Nobel lectures are downright scientific lectures, interspersed with curves, synoptic tables and quotations. I am somewhat reluctant to give here such a lecture on something that can be looked up in any modern schoolbook on physics. I will therefore not so much report here on physical and technical details and their connections but rather on the human experiences - some joyful events and many disappointments which had not been spared me and my colleagues on our way to the final breakthrough. This is not meant to be a complaint though; I rather feel that such experiences of scientists in quest of new approaches are absolutely understandable, or even normal. In such a representation I must, of course, consider the influence of my environment, in particular of my family. There have already been some scien- tists in my family: My father, Julius Ruska, was a historian of sciences in Heidelberg and Berlin; my uncle, Max Wolf, astronomer in Heidelberg; his assistant, a former pupil of my father and my godfather, August Kopff, Direc- tor of the Institute for astronomical calculation of the former Friedrich-Wil- helm University in Berlin. A cousin of my mother, Alfred Hoche, was Professor for Psychiatry in Freiburg/Breisgau; my grandfather from my mother’s side, Adalbert Merx, theologian in and Heidelberg. -

Discovery of the Cell and Mitosis
Discovery of the Cell and Mitosis While some scientists argued over spontaneous generation, others were using the first microscopes to examine and describe cells. The discovery of cells was only possible after the compound microscope was invented by a Dutch lens maker, Zacharias Janssen, in 1590. English physicist Robert Hooke first described cells in 1665. He made thin slices of cork (a type of tree) and observed many small boxes that reminded him of cells (small rooms) in a monastery. So, he called what he saw under the microscope “cells”. Because the cork was already dead and dried, the cells were empty. At the time Hooke thought they had only contained water when the cork was alive. In 1670, Antony van Leeuwenhoek, built a simple microscope that would magnify at 250x. He was the first person to observe bacteria and protozoa. He studied Protists, plant cells, various types of algae, and was the first person to view bacteria, which he termed "animalcules". Leeuwenhoek discovered these bacteria while viewing scrapings from his teeth and the teeth of others. He also discovered blood cells and was the first to see living sperm cells in animals. For the next 150 years, numerous scientists used both the simple and compound microscopes to look at many types of living and non-living materials. Barthelemy Dumortier was a botanist who was the first scientist to observe reproduction by cell division in plants. In 1832, he published his findings and called the process he saw “binary fission”. In 1838, Matthias Schleiden, a German botanist, concluded that all plant tissues are composed of cells and that an embryonic plant arose from a single cell. -

Heinrich Rohrer (1933–2013) Co-Inventor of the Scanning Tunnelling Microscope
COMMENT OBITUARY Heinrich Rohrer (1933–2013) Co-inventor of the scanning tunnelling microscope. einrich Rohrer, Heini to those who contemplate a new device. By 1981, the eventually verified by other groups and knew him, helped to open the door pair had designed the world’s first scanning presented at a workshop on the STM in the to nanotechnology. With Gerd tunnelling microscope (STM). Austrian Alps in 1985. Devices such as the HBinnig, he produced a device that allowed Unlike conventional microscopes, the atomic force microscope (AFM) — a very researchers to image and measure atoms STM did not use lenses. Instead, a probe high resolution type of scanning microscope and molecules, and to manipulate them. sharpened to a single atom at the tip was that measures the atomic forces between the Rohrer, who died on 16 May, tip of a probe and the surface being three weeks before his 80th scanned — have their roots in this birthday, was born in 1933, half meeting. During the last night of an hour after his twin sister. He the workshop, the mountains were grew up in the village of Buchs in abuzz with crazy ideas about how eastern Switzerland. Rohrer stud- such microscopes might be used ied physics at the Swiss Federal in applications in all sorts of fields, Institute of Technology (ETH) from fundamental physics and — ZURICH IBM RESEARCH in Zurich, where he remained to chemistry to information tech- pursue a PhD. It was during his nology, quantum computing and PhD years that he first came into molecular electronics, as well as in contact with the nanometre scale, the life sciences. -

Super Resolution Light Microscopy
Super Resolution Light Microscopy Wladimir Schaufler Nobel Prizes for Microscopy Developments Richard Zsigmondy invented the ultramicroscope. Nobel Price 1925 Frits Zernike invented the phase-contrast microscope. Nobel Price 1953 Maria Goeppert-Mayer described the two-photon excitation fluorescence. Nobel Price 1963 Ernst Ruska build the first electron microscope. Nobel Price 1986 Gerd Binnig designed the scanning tunneling microscope (STM). Nobel Price 1986 Nobel Prizes for using Microscopy as a Primary Scientific Instrument More than 10 Nobel Prizes, especially in the last 10 years including Harald zur Hausen, Nobel Prize for Physiology or Medicine, 2008 (1983 to 2003 DKFZ chairman). 11/20/2013 | Page 2 Wladimir Schaufler Super Resolution Light Microscopy Scale Issue 11/20/2013 | Page 3 Wladimir Schaufler Super Resolution Light Microscopy Optical Resolution (lateral) for Light Microscopy: Abbe Limit Numerical Aperture = n ·sin α Today best available objective: NA = 1.49 Source: Olympus Source: Abbe School of Photonics, Jena d 0,34 21,49 λ(Red Light) = 600nm ~210 nm Resolution Limit 11/20/2013 | Page 4 Wladimir Schaufler Super Resolution Light Microscopy Spatial Resolution of Biological Imaging Techniques http://zeiss-campus.magnet.fsu.edu/articles/superresolution/palm/practicalaspects.html 11/20/2013 | Page 5 Wladimir Schaufler Super Resolution Light Microscopy Localization Microscopy 11/20/2013 | Page 6 Wladimir Schaufler Super Resolution Light Microscopy Significantly Simplified Optical Setup for Localization Microscopy High Laser Power Density in Sample UV 405nm Blue 491nm Green 561nm Red 642nm DKFZ Super Resolution Laboratory (was established by W. Schaufler) at Central Microscopy Facility (Head: Dr. Felix Bestvater) 11/20/2013 | Page 7 Wladimir Schaufler Super Resolution Light Microscopy Localization Microscopy (SPDM) (developed and patented in the Prof. -
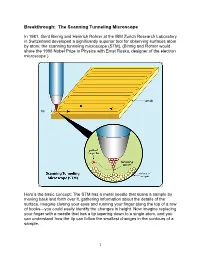
Breakthrough: the Scanning Tunneling Microscope
Breakthrough: The Scanning Tunneling Microscope In 1981, Gerd Binnig and Heinrich Rohrer at the IBM Zurich Research Laboratory in Switzerland developed a significantly superior tool for observing surfaces atom by atom: the scanning tunneling microscope (STM). (Binnig and Rohrer would share the 1986 Nobel Prize in Physics with Ernst Ruska, designer of the electron microscope.) Here’s the basic concept: The STM has a metal needle that scans a sample by moving back and forth over it, gathering information about the details of the surface. Imagine closing your eyes and running your finger along the top of a row of books—you could easily identify the changes in height. Now imagine replacing your finger with a needle that has a tip tapering down to a single atom, and you can understand how the tip can follow the smallest changes in the contours of a sample. 1 The needle doesn’t touch the sample, however, but stays about the width of two atoms above it. The STM takes advantage of what’s called the tunnel effect: If a voltage is applied to the tiny distance between the needle and the sample, electrons are able to tunnel, or jump, between the needle and the sample, creating an electric current. A computer receives the electrical signal and directs the needle to move up or down to keep the current constant—which keeps the distance between needle and sample constant. The path of the needle is recorded, and the computer can display that information as a grayscale image or topographical map. Scientists can add color to make the image easier to interpret. -

Nobel Prizes in Physics Closely Connected with the Physics of Solids
Nobel Prizes in Physics Closely Connected with the Physics of Solids 1901 Wilhelm Conrad Röntgen, Munich, for the discovery of the remarkable rays subsequently named after him 1909 Guglielmo Marconi, London, and Ferdinand Braun, Strassburg, for their contributions to the development of wireless telegraphy 1913 Heike Kamerlingh Onnes, Leiden, for his investigations on the properties of matter at low temperatures which lead, inter alia, to the production of liquid helium 1914 Max von Laue, Frankfort/Main, for his discovery of the diffraction of X-rays by crystals 1915 William Henry Bragg, London, and William Lawrence Bragg, Manchester, for their analysis of crystal structure by means of X-rays 1918 Max Planck, Berlin, in recognition of the services he rendered to the advancement of Physics by his discovery of energy quanta 1920 Charles Edouard Guillaume, Sèvres, in recognition of the service he has rendered to precise measurements in Physics by his discovery of anoma- lies in nickel steel alloys 1921 Albert Einstein, Berlin, for services to Theoretical Physics, and especially for his discovery of the law of the photoelectric effect 1923 Robert Andrews Millikan, Pasadena, California, for his work on the ele- mentary charge of electricity and on the photo-electric effect 1924 Manne Siegbahn, Uppsala, for his discoveries and researches in the field of X-ray spectroscopy 1926 Jean Baptiste Perrin, Paris, for his work on the discontinuous structure of matter, and especially for his discovery of sedimentation equilibrium 1928 Owen Willans Richardson, London, for his work on the thermionic phe- nomenon and especially for his discovery of the law named after him 1929 Louis Victor de Broglie, Paris, for his discovery of the wave nature of electrons 1930 Venkata Raman, Calcutta, for his work on the scattering of light and for the discovery of the effect named after him © Springer International Publishing Switzerland 2015 199 R.P. -

Appendix a I
Appendix A I Appendix A Professional Institutions and Associations AVA: Aerodynamische Versuchsanstalt (see under --+ KWIS) DFG: Deutsche Forschungs-Gemeinschaft (previously --+ NG) German Scientific Research Association. Full title: Deutsche Gemeinschaft zur Erhaltung und Forderung der Forschung (German Association for the Support and Advancement of Sci entific Research). Successor organization to the --+ NG, which was renamed DFG unofficially since about 1929 and officially in 1937. During the terms of its presidents: J. --+ Stark (June 1934-36); R. --+ Mentzel (Nov. 1936-39) and A. --+ Esau (1939-45), the DFG also had a dom inant influence on the research policy of the --+ RFR. It was funded by government grants in the millions and smaller contributions by the --+ Stifterverband. Refs.: ~1entzel [1940]' Stark [1943]c, Zierold [1968], Nipperdey & Schmugge [1970]. DGtP: Deutsche Gesellschaft fiir technische Physik German Society of Technical Physics. Founded on June 6, 1919 by Georg Gehlhoff as an alternative to the --+ DPG with a total of 13 local associations and its own journal --+ Zeitschrift fUr technische Physik. Around 1924 the DGtP had approximately 3,000 members, thus somewhat more than the DPG, but membership fell by 1945 to around 1,500. Chairmen: G. Gehlhoff (1920-31); K. --+ Mey (1931-45). Refs.: Gehlhoff et al. [1920]' Ludwig [1974], Richter [1977], Peschel (Ed.) [1991]' chap. 1, Heinicke [1985]' p. 43, Hoffmann & Swinne [1994]. DPG: Deutsche Physikalische Gesellschaft German Physical Society. Founded in 1899 a national organization at to succeed the Berlin Physical Society, which dates back to 1845. The Society issued regular biweekly proceedings, reports (Berichte) on the same, as well as the journal: Fortschritte der Physik (since 1845). -

James, Steinhauser, Hoffmann, Friedrich One Hundred Years at The
James, Steinhauser, Hoffmann, Friedrich One Hundred Years at the Intersection of Chemistry and Physics Published under the auspices of the Board of Directors of the Fritz Haber Institute of the Max Planck Society: Hans-Joachim Freund Gerard Meijer Matthias Scheffler Robert Schlögl Martin Wolf Jeremiah James · Thomas Steinhauser · Dieter Hoffmann · Bretislav Friedrich One Hundred Years at the Intersection of Chemistry and Physics The Fritz Haber Institute of the Max Planck Society 1911–2011 De Gruyter An electronic version of this book is freely available, thanks to the support of libra- ries working with Knowledge Unlatched. KU is a collaborative initiative designed to make high quality books Open Access. More information about the initiative can be found at www.knowledgeunlatched.org Aut ho rs: Dr. Jeremiah James Prof. Dr. Dieter Hoffmann Fritz Haber Institute of the Max Planck Institute for the Max Planck Society History of Science Faradayweg 4–6 Boltzmannstr. 22 14195 Berlin 14195 Berlin [email protected] [email protected] Dr. Thomas Steinhauser Prof. Dr. Bretislav Friedrich Fritz Haber Institute of the Fritz Haber Institute of the Max Planck Society Max Planck Society Faradayweg 4–6 Faradayweg 4–6 14195 Berlin 14195 Berlin [email protected] [email protected] Cover images: Front cover: Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry, 1913. From left to right, “factory” building, main building, director’s villa, known today as Haber Villa. Back cover: Campus of the Fritz Haber Institute of the Max Planck Society, 2011. The Institute’s his- toric buildings, contiguous with the “Röntgenbau” on their right, house the Departments of Physical Chemistry and Molecular Physics. -

On the History of Condensed Matter Physics� AIF - Pisa, February 2014
From Germanium to Graphene!: On the history of Condensed Matter Physics! AIF - Pisa, February 2014! A survey of Solid State Physics from 20-th to 21-th century:! a science that transformed the world around us! G. Grosso! February 18, 2014! Some considerations on a framework from which to grasp aspects and programs of fundamental and technological research in Condensed Matter Physics (CMP): a necessarily very incomplete account of condensed matter physics at the beginning of the 21th century. ! In the history of fundamental science, the area of Solid State Physics! represents the widest section of Physics and provides an example of! how Physics changes and what Physics can be.! In the 20-th century, research in Solid State Physics had enormous impact! both in basic aspects as well in technological applications.! Advances in ! - experimental techniques of measurements, ! - control of materials structures, ! - new theoretical concepts and numerical methods ! have been and actually are at the heart of this evolution.! Solid State Physics is at the root of most technologies of today’s world and! is a most clear evidence of how evolution of technology can be traced to! fundamental physics discoveries.! Just an example: Physics in communication industry…….! Eras of physics Communications technology changes Era of electromagnetism First electromagnet (1825) Electric currents<--> Magnetic fields (Oersted Telegraph systems (Cooke,Wheatstone, 1820, Faraday and Henry 1825) Morse 1837) Electromagnetic eq.s (Maxwell 1864), First transcontinental telegraph line (1861) e.m. waves propagation (Hertz 1880) Telephone (Bell 1874-76) Era of the electron Vacuum-tube diode (Fleming 1904)…… Discovery of the electron (Thomson, 1897) Wireless telegraph (Marconi 1896) Thermionic emission (Richardson 1901) Low energy electron diffraction (LEED) Wave nature of the electron (Davisson 1927) Radio astronomy (Jansky 1933) Era of quantum mechanics Kelly at Bell Labs.