Venom Characterization of the Three Species of Ophryacus and Proteomic Profiling of O. Sphenophrys Unveils Sphenotoxin, a Novel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Real-Time DNA Barcoding in a Remote Rainforest Using Nanopore Sequencing
bioRxiv preprint doi: https://doi.org/10.1101/189159; this version posted September 15, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Real-time DNA barcoding in a remote rainforest using nanopore sequencing 2 3 Aaron Pomerantz1,*, Nicolás Peñafiel2, Alejandro Arteaga3, Lucas Bustamante3, Frank Pichardo3, 4 Luis A. Coloma4, César L. Barrio-Amorós5, David Salazar-Valenzuela2, Stefan Prost 1,6,* 5 6 1 Department of Integrative Biology, University of California, Berkeley, CA, USA 7 2 Centro de Investigación de la Biodiversidad y Cambio Climático (BioCamb) e Ingeniería en 8 Biodiversidad y Recursos Genéticos, Facultad de Ciencias de Medio Ambiente, Universidad 9 Tecnológica Indoamérica, Machala y Sabanilla, Quito, Ecuador 10 3 Tropical Herping, Quito, Ecuador 11 4 Centro Jambatu de Investigación y Conservación de Anfibios, Fundación Otonga, Quito, 12 Ecuador 13 5 Doc Frog Expeditions, Uvita, Costa Rica 14 6 Program for Conservation Genomics, Department of Biology, Stanford University, Stanford, CA, 15 USA 16 17 * Corresponding authors: [email protected] (A. Pomerantz) and 18 [email protected] (S. Prost) 19 20 Abstract 21 Advancements in portable scientific instruments provide promising avenues to expedite 22 field work in order to understand the diverse array of organisms that inhabit our planet. 23 Here we tested the feasibility for in situ molecular analyses of endemic fauna using a 24 portable laboratory fitting within a single backpack, in one of the world’s most imperiled 25 biodiversity hotspots: the Ecuadorian Chocó rainforest. -

Crocodylus Moreletii
ANFIBIOS Y REPTILES: DIVERSIDAD E HISTORIA NATURAL VOLUMEN 03 NÚMERO 02 NOVIEMBRE 2020 ISSN: 2594-2158 Es un publicación de la CONSEJO DIRECTIVO 2019-2021 COMITÉ EDITORIAL Presidente Editor-en-Jefe Dr. Hibraim Adán Pérez Mendoza Dra. Leticia M. Ochoa Ochoa Universidad Nacional Autónoma de México Senior Editors Vicepresidente Dr. Marcio Martins (Artigos em português) Dr. Óscar A. Flores Villela Dr. Sean M. Rovito (English papers) Universidad Nacional Autónoma de México Editores asociados Secretario Dr. Uri Omar García Vázquez Dra. Ana Bertha Gatica Colima Dr. Armando H. Escobedo-Galván Universidad Autónoma de Ciudad Juárez Dr. Oscar A. Flores Villela Dra. Irene Goyenechea Mayer Goyenechea Tesorero Dr. Rafael Lara Rezéndiz Dra. Anny Peralta García Dr. Norberto Martínez Méndez Conservación de Fauna del Noroeste Dra. Nancy R. Mejía Domínguez Dr. Jorge E. Morales Mavil Vocal Norte Dr. Hibraim A. Pérez Mendoza Dr. Juan Miguel Borja Jiménez Dr. Jacobo Reyes Velasco Universidad Juárez del Estado de Durango Dr. César A. Ríos Muñoz Dr. Marco A. Suárez Atilano Vocal Centro Dra. Ireri Suazo Ortuño M. en C. Ricardo Figueroa Huitrón Dr. Julián Velasco Vinasco Universidad Nacional Autónoma de México M. en C. Marco Antonio López Luna Dr. Adrián García Rodríguez Vocal Sur M. en C. Marco Antonio López Luna Universidad Juárez Autónoma de Tabasco English style corrector PhD candidate Brett Butler Diseño editorial Lic. Andrea Vargas Fernández M. en A. Rafael de Villa Magallón http://herpetologia.fciencias.unam.mx/index.php/revista NOTAS CIENTÍFICAS SKIN TEXTURE CHANGE IN DIASPORUS HYLAEFORMIS (ANURA: ELEUTHERODACTYLIDAE) ..................... 95 CONTENIDO Juan G. Abarca-Alvarado NOTES OF DIET IN HIGHLAND SNAKES RHADINAEA EDITORIAL CALLIGASTER AND RHADINELLA GODMANI (SQUAMATA:DIPSADIDAE) FROM COSTA RICA ..... -

Atropoides Picadoi Agkistrodon Bilineatus
37 Beauty of the Beast SNAKES OF COSTA RICA AA DEADLYDEADLY CHARMCHARM Large and small, colorful or drab, harmless or dangerously venomous - meet some of the most fascinating ophidians of Mesoamerica 38 Spilotes pullatus Powerful, muscular, agile and fast-moving, this diurnal and highly variable species can attain a length of 2.6 meter / 8.5 feet. Relatively common in dry lowland riverine forest from Mexico to Argentina, it makes for an impressive encounter in the field, offering a most effective defensive display which includes mouth gaping, loud hissing and extreme inflating of the throat. 39 TEXTS BY POMPILIO CAMPOS BONILLA & ANDREA FERRARI PHOTOS BY ANDREA & ANTONELLA FERRARI and POMPILIO CAMPOS CHINCHILLA osta Rica is a Central American in major international treaties and Ctropical country which thanks to its conventions for the conservation of prevailing environmental conditions nature. A member of CITES, it follow can boast a rich diversity of snakes, strict rules regulating the international with a total of 11 families, 64 genera trade in endangered species - therefore and 139 ophidian species of aquatic, snakes enjoy benefits conferred by terrestrial and arboreal habits, law, ensuring their survival. However, distributed in almost all its territory, because of the myths and popular from sea level to an elevation of about beliefs about snakes, many species of 3000 meters. Only 22 of these possess great ecological importance are still a venom capable of causing harm to victims of human ignorance and are human health - these belong to the regularly killed, mainly in agricultural family Viperidae (pit vipers with heat- areas where workers are afraid of sensitive loreal pits, with haemotoxic being bitten. -

Inventario De Flora Y Fauna En El CBIMA (12.6
ii CRÉDITOS Comité Directivo José Vicente Troya Rodríguez Representante Residente del PNUD en Costa Rica Kryssia Brade Representante Residente Adjunta del PNUD en Costa Rica Coordinado por Miriam Miranda Quirós Coordinadora del proyecto Paisajes Productivos-PNUD Consultores que trabajaron en la realización del estudio José Esteban Jiménez, estudio de plantas vasculares Federico Oviedo Brenes, estudio de plantas vasculares Fabián Araya Yannarella, estudio de hongos Víctor J. Acosta-Chaves, estudios de aves, de anfibios y de reptiles Susana Gutiérrez Acuña, estudio de mamíferos Revisado por el comité editorial PNUD Rafaella Sánchez Ingrid Hernández Jose Daniel Estrada Diseño y diagramación Marvin Rojas San José, Costa Rica, 2019 iii RESUMEN EJECUTIVO El conocimiento sobre la diversidad 84 especies esperadas, 21 especies de biológica que habita los ecosistemas anfibios y 84 especies de reptiles. naturales, tanto boscosos como no boscosos, así como en áreas rurales y La diversidad acá presentada, con urbanas, es la línea base fundamental excepción de los hongos, es entre 100 y para establecer un manejo y una gestión 250% mayor respecto al estudio similar adecuadas sobre la protección, efectuado en el 2001 (FUNDENA 2001). conservación y uso sostenible de los La cantidad de especies sensibles es recursos naturales. En el Corredor baja para cada grupo de organismos Biológico Interurbano Maria Aguilar se respecto al total. El CBIMA posee una encontró un total de 765 especies de gran cantidad de especies de plantas plantas vasculares, (74.9% son nativas nativas con alto potencial para restaurar de Costa Rica y crecen naturalmente en espacios físicos degradados y para el CBIMA, un 3.5% son nativas de Costa utilizar como ornamentales. -

Squamata: Viperidae: Bothriechis) from the Costa Rican Highlands, with Notes on the Variation Within B
Zootaxa 4138 (2): 271–290 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4138.2.3 http://zoobank.org/urn:lsid:zoobank.org:pub:4F1E4A87-5370-420C-9C16-2BC373645110 A cryptic palm-pitviper species (Squamata: Viperidae: Bothriechis) from the Costa Rican highlands, with notes on the variation within B. nigroviridis TIFFANY M. DOAN1, ANDREW J. MASON1, TODD A. CASTOE2, MAHMOOD SASA3 & CHRISTOPHER L. PARKINSON1,4 1Department of Biology, University of Central Florida, 4000 Central Florida Blvd, Orlando, FL 32816, USA. E-mail: [email protected], [email protected], [email protected] 2Department of Biology, 501 S. Nedderman Drive, University of Texas at Arlington, Arlington, TX 76019, USA. E-mail: [email protected] 3Instituto Clodomiro Picado, Facultad de Microbiología, Universidad de Costa Rica, and Palo Verde Biological Station, Organization for Tropical Studies, San José, Costa Rica. E-mail: [email protected] 4Corresponding author Abstract Middle America is one of the most biodiverse regions in the world, harboring an exceptional number of rare and endemic species. This is especially true of Middle American cloud forests, where montane specialists occupy restricted, high-ele- vation ranges making them attractive candidates for investigating historical biogeography and speciation. One such high- land-restricted species, the black speckled palm-pitviper (Bothriechis nigroviridis), occupies the Central, Tilarán, and Talamanca Cordilleras in Costa Rica and Panama. In this study, we investigate the genetic and morphological variation among populations of B. nigroviridis by inferring a multilocus phylogeny (21 individuals) and analyzing meristic scale characters with a principal component analysis (64 individuals). -

Tan Choo Hock Thesis Submitted in Fulfi
TOXINOLOGICAL CHARACTERIZATIONS OF THE VENOM OF HUMP-NOSED PIT VIPER (HYPNALE HYPNALE) TAN CHOO HOCK THESIS SUBMITTED IN FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY FACULTY OF MEDICINE UNIVERSITY OF MALAYA KUALA LUMPUR 2013 Abstract Hump-nosed pit viper (Hypnale hypnale) is a medically important snake in Sri Lanka and Western Ghats of India. Envenomation by this snake still lacks effective antivenom clinically. The species is also often misidentified, resulting in inappropriate treatment. The median lethal dose (LD50) of H. hypnale venom varies from 0.9 µg/g intravenously to 13.7 µg/g intramuscularly in mice. The venom shows procoagulant, hemorrhagic, necrotic, and various enzymatic activities including those of proteases, phospholipases A2 and L-amino acid oxidases which have been partially purified. The monovalent Malayan pit viper antivenom and Hemato polyvalent antivenom (HPA) from Thailand effectively cross-neutralized the venom’s lethality in vitro (median effective dose, ED50 = 0.89 and 1.52 mg venom/mL antivenom, respectively) and in vivo in mice, besides the procoagulant, hemorrhagic and necrotic effects. HPA also prevented acute kidney injury in mice following experimental envenomation. Therefore, HPA may be beneficial in the treatment of H. hypnale envenomation. H. hypnale-specific antiserum and IgG, produced from immunization in rabbits, effectively neutralized the venom’s lethality and various toxicities, indicating the feasibility to produce an effective specific antivenom with a common immunization regime. On indirect ELISA, the IgG cross-reacted extensively with Asiatic crotalid venoms, particularly that of Calloselasma rhodostoma (73.6%), suggesting that the two phylogenically related snakes share similar venoms antigenic properties. -

Ecography E6281 Daza, J
Ecography E6281 Daza, J. M., Castoe, C. L. and Parkinson, C. L. 2010. Using regional comparative phylogeographic data from snake lineages to infer historical processes in Middle America. – Ecography 33: 343–354. Supplementary material Table S1. Genbank sequences utilized in this study. Taxa Locality Voucher Cyt-b ND4 Agkistrodon bilineatus Costa Rica, Guanacaste WWL AY223613 AF156585 Agkistrodon contortrix USA, Ohio, Athens Co. Moody 338 AY223612 AF156576 Agkistrodon piscivorus USA, South Carolina CLP-30 AY223615 AF156578 Agkistrodon taylori Mexico, Tamaulipas CLP-140 AY223614 AF156580 Atractus lasallei Colombia, Antioquia MHUA 14368 GQ334480 GQ334581 Atropoides indomitus Honduras, Olancho ENS-10630 DQ061194 DQ061219 Atropoides mexicanus Costa Rica, San Jose CLP-168 AY223584 U41871 Atropoides nummifer Mexico, Puebla, ENS-10515 DQ061195 DQ061220 Atropoides occiduus Guatemala, Escuintla UTA-R-29680 AY220315 AY220338 Atropoides olmec Mexico, Veracruz UTA-R-14233 AY220322 AY220345 Atropoides picadoi Costa Rica, Alajuela, Varablanca CLP-45 AY223593 U41872 Bothriechis aurifer Guatemala UTA-R35031 DQ305466 DQ305483 Bothriechis bicolor UTA-R34156 DQ305467 DQ305484 Bothriechis lateralis Costa Rica, Acosta MZUCR-11155 AY223588 U41873 Bothriechis marchi Guatemala, Zacapa, Cerro del Mono UTA-R52959 DQ305469 DQ305486 Bothriechis nigroviridis Costa Rica, San Gerondo de Dota MZUCR-11151 AY223589 AY223635 Bothriechis rowleyi Mexico, Cerro Baúl JAC 13295 DQ305468 DQ305485 Bothriechis schlegelii Costa Rica, Cariblanco de Sarapiquí MZUCR-11149 AY223590 AY223636 -

An Updated Review of the Pythons Including Resolution of Issues of Taxonomy and Nomenclature
2 Australasian Journal of Herpetology Australasian Journal of herpetology 10: 2-32. ISSN 1836-5698 (Print) Published 8 April 2012. ISSN 1836-5779 (Online) AN UPDATED REVIEW OF THE PYTHONS INCLUDING RESOLUTION OF ISSUES OF TAXONOMY AND NOMENCLATURE. RAYMOND T. HOSER 488 Park Road, Park Orchards, Victoria, 3134, Australia. Phone: +61 3 9812 3322 Fax: 9812 3355 E-mail: [email protected] Received 27 March 2012, Accepted 5 April 2012, Published 8 April 2012. ABSTRACT This paper reviews the python group of snakes. It resolves issues of taxonomy and nomenclature, including by means of publication (this paper), effectively settling any disputes about potential validity of names for use according to the ICZN rules for various well-defined taxa. In accordance with the ICZN code, this paper formally names one new genus (Jackypython gen. nov.), one new subgenus (Rawlingspython subgen. nov), two new species, (Morelia wellsi sp. nov. and Australiasis funki sp. nov.) and one new subspecies, (Chondropython viridis adelynhoserae subsp. nov.). A neotype is desig- nated for A. amethistina. Furthermore, four subspecies within the genus Aspidites and one subspecies within Leiopython are formally named. Assessed are matters relating to the genus Leiopython and a 2008 paper by Wulf Schleip. This paper redefines the family composition at tribe level. As a result, one new tribe is erected, namely Broghammerini tribe nov.. For the pre-existing tribe Moreliini there are four newly identified subtribes, namely Moreliina subtribe nov., Aspiditesina subtribe nov., -

Body Size Distributions at Community, Regional Or Taxonomic Scales Do Not
Global Ecology and Biogeography, (Global Ecol. Biogeogr.) (2013) bs_bs_banner RESEARCH Body size distributions at local, PAPER community or taxonomic scales do not predict the direction of trait-driven diversification in snakes in the United States Frank T. Burbrink1,2* and Edward A. Myers1,2 1Department of Biology, The College of Staten ABSTRACT Island, The City University of New York, 2800 Aim We determine whether trait-driven diversification yields similar body size Victory Boulevard, Staten Island, NY 10314, USA, 2Department of Biology, The Graduate distributions for snakes in local, regional and phylogenetic assemblages. School and University Center, The City Location United States, North America. University of New York, 365 Fifth Avenue, New York, NY 10016, USA Methods Using total length and mass, we examine body size frequency distribu- tions (BSFD) across 79 sites and respective biomes to determine if these areas represent random subsamples from the source pools of taxon body sizes. Using QuaSSE, we determine if the most probable model of trait-driven diversification in the three most common groups of snakes in North America, the ratsnakes, pitvipers and watersnakes, is similar to the predicted regional BSFD. Results BSFD of snakes at the community, biome, regional and clade scales show symmetric distributions of body size. These patterns may simply be generated from random statistical subsampling. Speciation rates are not highest at or near the modal body size and simulations show that linear trait-driven models can still yield highly symmetric distributions of body size. Main conclusions In this study region, processes such as competition due to size do not alter BSFD from one scale to the other. -

Denisonia Hydrophis Parapistocalamus Toxicocalamus Disteira Kerilia Pelamis Tropidechis Drysdalia Kolpophis Praescutata Vermicella Echiopsis Lapemis
The following is a work in progress and is intended to be a printable quick reference for the venomous snakes of the world. There are a few areas in which common names are needed and various disputes occur due to the nature of such a list, and it will of course be continually changing and updated. And nearly all species have many common names, but tried it simple and hopefully one for each will suffice. I also did not include snakes such as Heterodon ( Hognoses), mostly because I have to draw the line somewhere. Disclaimer: I am not a taxonomist, that being said, I did my best to try and put together an accurate list using every available resource. However, it must be made very clear that a list of this nature will always have disputes within, and THIS particular list is meant to reflect common usage instead of pioneering the field. I put this together at the request of several individuals new to the venomous endeavor, and after seeing some very blatant mislabels in the classifieds…I do hope it will be of some use, it prints out beautifully and I keep my personal copy in a three ring binder for quick access…I honestly thought I knew more than I did…LOL… to my surprise, I learned a lot while compiling this list and I hope you will as well when you use it…I also would like to thank the following people for their suggestions and much needed help: Dr.Wolfgang Wuster , Mark Oshea, and Dr. Brian Greg Fry. -

Snake Venomics and Antivenomics of Bothrops Diporus, a Medically Important Pitviper in Northeastern Argentina
Article Snake Venomics and Antivenomics of Bothrops diporus, a Medically Important Pitviper in Northeastern Argentina Carolina Gay 1,†, Libia Sanz 2, Juan J. Calvete 2,* and Davinia Pla 2,*,† Received: 17 November 2015; Accepted: 17 December 2015; Published: 25 December 2015 Academic Editor: Stephen P. Mackessy 1 Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste, Avenida Libertad 5470, 3400 Corrientes, Argentina; [email protected] 2 Instituto de Biomedicina de Valencia, CSIC, Jaime Roig 11, 46010 Valencia, Spain; [email protected] * Correspondence: [email protected] (J.J.C.); [email protected] (D.P.); Tel.: +34-96-339-1778 (J.J.C. & D.P.); Fax: +34-96-369-0800 (J.J.C. & D.P.) † These authors contributed equally to this work. Abstract: Snake species within genus Bothrops are responsible for more than 80% of the snakebites occurring in South America. The species that cause most envenomings in Argentina, B. diporus, is widely distributed throughout the country, but principally found in the Northeast, the region with the highest rates of snakebites. The venom proteome of this medically relevant snake was unveiled using a venomic approach. It comprises toxins belonging to fourteen protein families, being dominated by PI- and PIII-SVMPs, PLA2 molecules, BPP-like peptides, L-amino acid oxidase and serine proteinases. This toxin profile largely explains the characteristic pathophysiological effects of bothropic snakebites observed in patients envenomed by B. diporus. Antivenomic analysis of the SAB antivenom (Instituto Vital Brazil) against the venom of B. diporus showed that this pentabothropic antivenom efficiently recognized all the venom proteins and exhibited poor affinity towards the small peptide (BPPs and tripeptide inhibitors of PIII-SVMPs) components of the venom. -
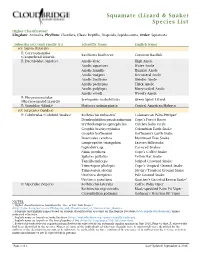
Squamate (Lizard & Snake) Species List
Squamate (Lizard & Snake) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Lepidosauria, Order: Squamata Suborder (sO:) and Family (F:) Scientific Name2 English Name2 sO: Sauria (Lizards) F: Corytophanidae Basiliscus basiliscus Common Basilisk (Casquehead Lizards) F: Dactyloidae (Anoles) Anolis altae High Anole Anolis aquaticus Water Anole Anolis humilis Humble Anole Anolis insignis Decorated Anole Anolis limifrons Slender Anole Anolis pachypus Thick Anole Anolis polylepis Many-scaled Anole Anolis woodi Wood's Anole F: Phrynosomatidae Sceloporus malachiticus Green Spiny Lizard (Phrynosomatid Lizards) F: Scincidae (Skinks) Marisora unimarginata Central American Mabuya sO: Serpentes (Snakes) F: Colubridae (Colubrid Snakes) Bothriechis nubestris5 Talamancan Palm-Pitviper5 Dendrophidion paucicarinatum Cope's Forest Racer Erythrolamprus epinephelus Culebra boba verde Geophis brachycephalus Colombian Earth Snake Geophis hoffmanni Hoffmann's Earth Snake Imantodes cenchoa Blunthead Tree Snake Lampropeltis triangulum Eastern Milksnake Leptodeira sp. Cat-eyed Snakes Ninia psephota Cope's Coffee Snake Spilotes pullatus Yellow Rat Snake Tantilla ruficeps Striped Crowned Snake3 Trimetopon pliolepis Cope's Tropical Ground Snake Trimetopon slevini Slevin's Tropical Ground Snake Urotheca decipiens Pale Ground Snake Urotheca guentheri Gunther's Graceful Brown Snake4 F: Viperidae (Vipers) Bothriechis lateralis Coffee Palm Viper Bothriechis nigroviridis5 Black-speckled Palm Pit Viper5 Cerrophidion godmani Godman's Montane Pit Viper NOTES: 1, Higher classification as found on the Tree of Life Web Project (http://tolweb.org/accessory/Phylogeny_and_Classification_of_Amniotes?acc_id=462). 2, Scientific and English names based on current classifications as found on The Reptile Database (www.reptile-database.org), unless indicated otherwise. 3, English name as found on SnakeDatabase (http://snakedatabase.org/species/tantilla/ruficeps). 4, English name as found on The Encyclopedia of Life (http://eol.org/pages/1055095/overview).