Vaccine Information for PARENTS and CAREGIVERS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

(MMRV) Vaccine
Addendum to MMR Vaccine (Measles, Mumps, Rubella, and Varicella): What You Need to Know Vaccine Information Statement 1. I agree that the person named below will get the vaccine checked below. 2. I received or was offered a copy of the Vaccine Information Statement (VIS) for the vaccine listed above. 3. I know the risks of the disease this vaccine prevents. 4. I know the benefits and risks of the vaccine. 5. I have had a chance to ask questions about the disease the vaccine prevents, the vaccine, and how the vaccine is given. 6. I know that the person named below will have the vaccine put in his/her body to prevent the disease this vaccine prevents. 7. I am an adult who can legally consent for the person named below to get the vaccine. I freely and voluntarily give my signed permission for this vaccine. Vaccine to be given: Measles, Mumps, Rubella, and Varicella (MMRV) Vaccine Information about person to receive vaccine (Please print) Name: Last First Middle Initial Birthdate Sex (mm/dd/yy) (circle one) M F Address: Street City County State Zip TX Signature of person to receive vaccine or person authorized to make the request (parent or guardian): x Date: x Date: Witness PRIVACY NOTIFICATION - With few exceptions, you have the right to request and be informed about information that the State of Texas collects about you. You are entitled to receive and review the information upon request. You also have the right to ask the state agency to correct any information that is determined to be incorrect. -

Safety of Immunization During Pregnancy a Review of the Evidence
Safety of Immunization during Pregnancy A review of the evidence Global Advisory Committee on Vaccine Safety © World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications –whether for sale or for non-commercial distribution– should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. -

Mmrv Vaccine
VACCINE INFORMATION STATEMENT (Measles, Mumps, Many Vaccine Information Statements are available in Spanish and other languages. MMRV Vaccine Rubella and See www.immunize.org/vis Varicella) Hojas de información sobre vacunas están disponibles en español y en muchos otros What You Need to Know idiomas. Visite www.immunize.org/vis These are recommended ages. But children can get the Measles, Mumps, Rubella and second dose up through 12 years as long as it is at least 1 Varicella 3 months after the first dose. Measles, Mumps, Rubella, and Varicella (chickenpox) can be serious diseases: Children may also get these vaccines as 2 separate shots: MMR (measles, mumps and rubella) and Measles varicella vaccines. • Causes rash, cough, runny nose, eye irritation, fever. • Can lead to ear infection, pneumonia, seizures, brain 1 Shot (MMRV) or 2 Shots (MMR & Varicella)? damage, and death. • Both options give the same protection. Mumps • One less shot with MMRV. • Causes fever, headache, swollen glands. • Children who got the first dose as MMRV have • Can lead to deafness, meningitis (infection of the brain had more fevers and fever-related seizures (about and spinal cord covering), infection of the pancreas, 1 in 1,250) than children who got the first dose as painful swelling of the testicles or ovaries, and, rarely, separate shots of MMR and varicella vaccines on death. the same day (about 1 in 2,500). Rubella (German Measles) Your doctor can give you more information, • Causes rash and mild fever; and can cause arthritis, including the Vaccine Information Statements for (mostly in women). MMR and Varicella vaccines. -

Vaccines and Autism: What You Should Know | Vaccine Education
Q A Vaccines and Autism: What you should know Volume& 1 Summer 2008 Some parents of children with autism are concerned that vaccines are the cause. Their concerns center on three areas: the combination measles-mumps-rubella (MMR) vaccine; thimerosal, a mercury-containing preservative previously contained in several vaccines; and the notion that babies receive too many vaccines too soon. Q. What are the symptoms of autism? Q. Does the MMR vaccine cause autism? A. Symptoms of autism, which typically appear during the A. No. In 1998, a British researcher named Andrew Wakefi eld fi rst few years of life, include diffi culties with behavior, social raised the notion that the MMR vaccine might cause autism. skills and communication. Specifi cally, children with autism In the medical journal The Lancet, he reported the stories of may have diffi culty interacting socially with parents, siblings eight children who developed autism and intestinal problems and other people; have diffi culty with transitions and need soon after receiving the MMR vaccine. To determine whether routine; engage in repetitive behaviors such as hand fl apping Wakefi eld’s suspicion was correct, researchers performed or rocking; display a preoccupation with activities or toys; a series of studies comparing hundreds of thousands of and suffer a heightened sensitivity to noise and sounds. children who had received the MMR vaccine with hundreds Autism spectrum disorders vary in the type and severity of of thousands who had never received the vaccine. They found the symptoms they cause, so two children with autism may that the risk of autism was the same in both groups. -

Proquad, INN-Measles, Mumps, Rubella and Varicella Vaccine (Live)
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT ProQuad powder and solvent for suspension for injection ProQuad powder and solvent for suspension for injection in a pre-filled syringe Measles, mumps, rubella and varicella vaccine (live). 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, one dose (0.5 mL) contains: 1 Measles virus Enders’ Edmonston strain (live, attenuated) ........ not less than 3.00 log10 TCID * 50 1 Mumps virus Jeryl Lynn™ (Level B) strain (live, attenuated) ... not less than 4.30 log10 TCID * 50 2 Rubella virus Wistar RA 27/3 strain (live, attenuated)............... not less than 3.00 log10 TCID * 50 3 Varicella virus Oka/Merck strain (live, attenuated) ................... not less than 3.99 log10 PFU** *50% tissue culture infectious dose **plaque-forming units (1) Produced in chick embryo cells. (2) Produced in human diploid lung (WI-38) fibroblasts. (3) Produced in human diploid (MRC-5) cells. The vaccine may contain traces of recombinant human albumin (rHA). This vaccine contains a trace amount of neomycin. See section 4.3. Excipient(s) with known effect The vaccine contains 16 milligrams of sorbitol per dose. See section 4.4. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder and solvent for suspension for injection Before reconstitution, the powder is a white to pale yellow compact crystalline cake and the solvent is a clear colourless liquid. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications ProQuad is indicated for simultaneous vaccination against measles, mumps, rubella and varicella in individuals from 12 months of age. ProQuad can be administered to individuals from 9 months of age under special circumstances (e.g., to conform with national vaccination schedules, outbreak situations, or travel to a region with high prevalence of measles; see sections 4.2, 4.4, and 5.1). -

Frequently Asked Questions About Measles Immunizations
Immunization Unit Assessment, Compliance, and Evaluation Group Measles FAQs Q: When did the vaccine for measles become available? A: The first measles vaccines were available in 1963, and were replaced with a much better vaccine in 1968. Q: Do people who received MMR in the 1960s need to have their dose repeated? A: Not necessarily. People who have documentation of receiving a live measles vaccine in the 1960s do not need to be revaccinated. People who were vaccinated with a killed or inactivated measles vaccine, or vaccine of unknown type, should be revaccinated with at least 1 dose of MMR. Q: What kind of vaccine is it? A: The vaccines available in the United States that protect against measles are the MMR and MMRV, which protects against measles, mumps, and rubella or measles, mumps, rubella, and varicella (chickenpox). They are both live attenuated, or weakened, vaccines. Q: Which vaccine can I get, the MMR or the MMRV? A: The MMRV is only approved for people aged 12 months – 12 years. The MMR is approved for anyone 6 months of age and older, but it is routinely recommended for use in people 12 months of age and older. Any dose of MMR received before 12 months of age is not considered valid and should be repeated in 4 weeks or at age 12 months, whichever is later. Q: How is the vaccine given? A: The vaccine is administered in the fatty layer of tissue underneath the skin. Q: Is the measles shot just a one-time dose? A: The measles vaccine is very effective. -

Summary of the WHO Position on Rubella Vaccine- July 2020 This
Summary of the WHO position on Rubella Vaccine- July 2020 This document replaces the WHO position paper on rubella vaccine published in the Weekly Epidemiological Record in July 2011. It incorporates the most recent developments in the field of rubella vaccines to provide updated guidance on the introduction and use of rubella-containing vaccines (RCVs) in national immunization schedules. It specifically updates guidance on co- administration of rubella vaccine with yellow fever (YF) vaccine and updates data and the WHO position on the control and elimination of rubella. Background Rubella is of public health importance because of the teratogenic potential of infections acquired during pregnancy. Rubella virus is generally recognized as the most common infectious cause of birth defects, accounting for an estimated 100 000 infants born with congenital rubella syndrome (CRS) each year worldwide. Infection with rubella virus within 12 days of conception and early pregnancy (usually within the first 8–10 weeks) may result in miscarriage, fetal death or CRS. For the rest of the population, rubella is an acute, usually mild viral infection transmitted by respiratory droplets, which affects susceptible children and adults worldwide. A number of live, attenuated rubella vaccines are currently available. Most rubella vaccines are available in combination with other vaccine antigens such as measles, mumps and varicella (MR, MMR, MMRV, respectively) and also in a monovalent formulation. WHO position In light of the global burden of CRS, the proven efficacy, effectiveness and safety of RCVs and regional elimination goals, WHO recommends that countries introduce RCVs into their national immunization programmes. This recommendation includes the opportunity offered by accelerated measles elimination activities to achieve both goals. -

Measles: Chapter 7.1 Chapter 7: Measles Paul A
VPD Surveillance Manual 7 Measles: Chapter 7.1 Chapter 7: Measles Paul A. Gastanaduy, MD, MPH; Susan B. Redd; Nakia S. Clemmons, MPH; Adria D. Lee, MSPH; Carole J. Hickman, PhD; Paul A. Rota, PhD; Manisha Patel, MD, MS I. Disease Description Measles is an acute viral illness caused by a virus in the family paramyxovirus, genus Morbillivirus. Measles is characterized by a prodrome of fever (as high as 105°F) and malaise, cough, coryza, and conjunctivitis, followed by a maculopapular rash.1 The rash spreads from head to trunk to lower extremities. Measles is usually a mild or moderately severe illness. However, measles can result in complications such as pneumonia, encephalitis, and death. Approximately one case of encephalitis2 and two to three deaths may occur for every 1,000 reported measles cases.3 One rare long-term sequelae of measles virus infection is subacute sclerosing panencephalitis (SSPE), a fatal disease of the central nervous system that generally develops 7–10 years after infection. Among persons who contracted measles during the resurgence in the United States (U.S.) in 1989–1991, the risk of SSPE was estimated to be 7–11 cases/100,000 cases of measles.4 The risk of developing SSPE may be higher when measles occurs prior to the second year of life.4 The average incubation period for measles is 11–12 days,5 and the average interval between exposure and rash onset is 14 days, with a range of 7–21 days.1, 6 Persons with measles are usually considered infectious from four days before until four days after onset of rash with the rash onset being considered as day zero. -

Immunization-Form.Pdf
James Madison University Immunization Form COMMONWEALTH OF VIRGINIA LAW REQUIRES THAT THE CERTIFICATE OF IMMUNIZATION AND TB SCREENING BE COMPLETED AND SUBMITTED TO THE UNIVERSITY HEALTH CENTER. Instructions for new students: 1) Download (if .pdf does not display correctly, open the file in Adobe Reader) and print the Immunization Form and have it completed and signed by a health care professional. An official immunization record from your doctor or another school will be accepted. 2) Log into your MyJMUChart account to upload the completed and signed immunization form (or official record), as well as a copy of your health insurance card (front and back.) All uploaded forms must be in .pdf format. Immunizations must be up to date. 3) Complete the required TB Assessment and Health History for NEW students located under the “forms” tab in MyJMUChart. Due dates for undergraduate students: July 8, 2021 for Fall 2021 semester start and December 10, 2021 for Spring 2022 start. Due date for graduate students: No later than the third Friday of the first semester attending JMU. An enrollment hold and a $50 fine will be placed on your account if your immunization form and TB Screening are not deemed complete by the Health Center staff. CERTIFICATE OF IMMUNIZATION* This MUST be signed by a health care provider Name (print): _______________________________________________ Date of Birth: ______/_______/_______________ Date completed: _______/_______/_______ STUDENT ID NUMBER: __________________________ REQUIRED IMMUNIZATIONS COVID-19 (indicate which -
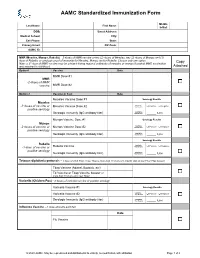
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Combination Vaccines
INFORMATION FOR PARENTS Combination Vaccines Last updated April 2014 The measles, mumps, and rubella vaccine (MMR) and Combination vaccines reduce the diphtheria, tetanus, and pertussis vaccine (DTaP) each protect your child against three diseases. However, these number of shots your child needs two vaccines are not considered true combination vaccines while protecting against several because in the United States, you cannot get separate serious diseases. vaccines for all of the diseases that MMR and DTaP protect against. Fewer Shots—Same Protection Some examples of common combination vaccines for children are: Combination vaccines take two or more vaccines that could be given individually and put them into one • Comvax, which combines Hib and Hep B shot. Children get the same protection as they do from • Twinrix, which combines Hep A and Hep B individual vaccines given separately—but with fewer shots. • Pediarix, which combines DTaP, Hep B, and IPV (polio) So, at a doctor’s visit, your child may only get two or three shots to protect him from five diseases, instead of five • ProQuad, which combines MMR and individual shots. Fewer shots may mean less pain for your varicella (chickenpox) child and less stress for you. • Kinrix, which combines DTaP and IPV (polio) • Pentacel, which combines DTaP, IPV (polio), and Hib To learn about the diseases that vaccines prevent, visit: http://www.cdc.gov/vaccines/vpd-vac/fact-sheet-parents.html Fewer Shots—On Time Protection Combination vaccines help parents, doctors, and nurses keep children up-to-date on vaccines. Combining vaccines into fewer shots may mean that more children will get recommended vaccinations on time.