Cells, Tissues and Organs of the Immune System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1
2912 Vol. 8, 2912–2923, September 2002 Clinical Cancer Research Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1 Jennifer A. Tuxhorn, Gustavo E. Ayala, Conclusions: The stromal microenvironment in human Megan J. Smith, Vincent C. Smith, prostate cancer is altered compared with normal stroma and Truong D. Dang, and David R. Rowley2 exhibits features of a wound repair stroma. Reactive stroma is composed of myofibroblasts and fibroblasts stimulated to Departments of Molecular and Cellular Biology [J. A. T., T. D. D., express extracellular matrix components. Reactive stroma D. R. R.] and Pathology [G. E. A., M. J. S., V. C. S.] Baylor College of Medicine, Houston, Texas 77030 appears to be initiated during PIN and evolve with cancer progression to effectively displace the normal fibromuscular stroma. These studies and others suggest that TGF-1isa ABSTRACT candidate regulator of reactive stroma during prostate can- Purpose: Generation of a reactive stroma environment cer progression. occurs in many human cancers and is likely to promote tumorigenesis. However, reactive stroma in human prostate INTRODUCTION cancer has not been defined. We examined stromal cell Activation of the host stromal microenvironment is pre- phenotype and expression of extracellular matrix compo- dicted to be a critical step in adenocarcinoma growth and nents in an effort to define the reactive stroma environ- progression (1–5). Several human cancers have been shown to ment and to determine its ontogeny during prostate cancer induce a stromal reaction or desmoplasia as a component of progression. carcinoma progression. However, the specific mechanisms of Experimental Design: Normal prostate, prostatic intra- stromal cell activation are not known, and the extent to which epithelial neoplasia (PIN), and prostate cancer were exam- stroma regulates the biology of tumorigenesis is not fully un- ined by immunohistochemistry. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates
Research Article Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates David Gius,1 Margo C. Funk,2 Eric Y. Chuang,1 Sheng Feng,3 Phyllis C. Huettner,4 Loan Nguyen,2 C. Matthew Bradbury,1 Mark Mishra,1 Shuping Gao,1 Barbara M. Buttin,2 David E. Cohn,2 Matthew A. Powell,2 Neil S. Horowitz,2 Bradford P. Whitcomb,2 and JanetS. Rader 2 1Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Maryland and 2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology; 3Division of Biostatistics; and 4Lauren V. Ackerman Laboratory of Surgical Pathology, Washington University School of Medicine, St. Louis, Missouri Abstract (CIN 1–3) and finally to squamous cell carcinoma antigen (SCCA) This study is the first comprehensive, integrated approach to have been well characterized. Histologically, CIN 1 consists of examine grade-specific changes in gene expression along the immature basal-type cells involving the lower third of the entire neoplastic spectrum of cervical intraepithelial neoplasia epithelium. In CIN 2, these immature basal-type cells involve more (CIN) in the process of cervical carcinogenesis. This was than the lower third, whereas CIN 3 involves the full thickness of the accomplished by identifying gene expression signatures of epithelium. In addition, higher CIN grades exhibit nuclear crowding, disease progression using cDNA microarrays to analyze RNA pleomorphism, loss of cell polarity, and increased mitotic activity from laser-captured microdissected epithelium and underlying (1). These transitions seemto be well conserved and, as such, stroma from normal cervix, graded CINs, cancer, and patient- provide an intriguing systemto use genomicsto identify the early matched normal cervical tissues. -

Colposcopy of the Uterine Cervix
THE CERVIX: Colposcopy of the Uterine Cervix • I. Introduction • V. Invasive Cancer of the Cervix • II. Anatomy of the Uterine Cervix • VI. Colposcopy • III. Histology of the Normal Cervix • VII: Cervical Cancer Screening and Colposcopy During Pregnancy • IV. Premalignant Lesions of the Cervix The material that follows was developed by the 2002-04 ASCCP Section on the Cervix for use by physicians and healthcare providers. Special thanks to Section members: Edward J. Mayeaux, Jr, MD, Co-Chair Claudia Werner, MD, Co-Chair Raheela Ashfaq, MD Deborah Bartholomew, MD Lisa Flowers, MD Francisco Garcia, MD, MPH Luis Padilla, MD Diane Solomon, MD Dennis O'Connor, MD Please use this material freely. This material is an educational resource and as such does not define a standard of care, nor is intended to dictate an exclusive course of treatment or procedure to be followed. It presents methods and techniques of clinical practice that are acceptable and used by recognized authorities, for consideration by licensed physicians and healthcare providers to incorporate into their practice. Variations of practice, taking into account the needs of the individual patient, resources, and limitation unique to the institution or type of practice, may be appropriate. I. AN INTRODUCTION TO THE NORMAL CERVIX, NEOPLASIA, AND COLPOSCOPY The uterine cervix presents a unique opportunity to clinicians in that it is physically and visually accessible for evaluation. It demonstrates a well-described spectrum of histological and colposcopic findings from health to premalignancy to invasive cancer. Since nearly all cervical neoplasia occurs in the presence of human papillomavirus infection, the cervix provides the best-defined model of virus-mediated carcinogenesis in humans to date. -

Histology Histology
HISTOLOGY HISTOLOGY ОДЕСЬКИЙ НАЦІОНАЛЬНИЙ МЕДИЧНИЙ УНІВЕРСИТЕТ THE ODESSA NATIONAL MEDICAL UNIVERSITY Áiáëiîòåêà ñòóäåíòà-ìåäèêà Medical Student’s Library Серія заснована в 1999 р. на честь 100-річчя Одеського державного медичного університету (1900–2000 рр.) The series is initiated in 1999 to mark the Centenary of the Odessa State Medical University (1900–2000) 1 L. V. Arnautova O. A. Ulyantseva HISTÎLÎGY A course of lectures A manual Odessa The Odessa National Medical University 2011 UDC 616-018: 378.16 BBC 28.8я73 Series “Medical Student’s Library” Initiated in 1999 Authors: L. V. Arnautova, O. A. Ulyantseva Reviewers: Professor V. I. Shepitko, MD, the head of the Department of Histology, Cytology and Embryology of the Ukrainian Medical Stomatologic Academy Professor O. Yu. Shapovalova, MD, the head of the Department of Histology, Cytology and Embryology of the Crimean State Medical University named after S. I. Georgiyevsky It is published according to the decision of the Central Coordinational Methodical Committee of the Odessa National Medical University Proceedings N1 from 22.09.2010 Навчальний посібник містить лекції з гістології, цитології та ембріології у відповідності до програми. Викладено матеріали теоретичного курсу по всіх темах загальної та спеціальної гістології та ембріології. Посібник призначений для підготовки студентів до практичних занять та ліцензійного екзамену “Крок-1”. Arnautova L. V. Histology. A course of lectures : a manual / L. V. Arnautova, O. A. Ulyantseva. — Оdessa : The Оdessa National Medical University, 2010. — 336 p. — (Series “Medical Student’s Library”). ISBN 978-966-443-034-7 The manual contains the lecture course on histology, cytology and embryol- ogy in correspondence with the program. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Tumor-Associated Stromal Cells As Key Contributors to the Tumor Microenvironment Karen M
Bussard et al. Breast Cancer Research (2016) 18:84 DOI 10.1186/s13058-016-0740-2 REVIEW Open Access Tumor-associated stromal cells as key contributors to the tumor microenvironment Karen M. Bussard1,2, Lysette Mutkus3, Kristina Stumpf3, Candelaria Gomez-Manzano4 and Frank C. Marini1,3* Abstract The tumor microenvironment is a heterogeneous population of cells consisting of the tumor bulk plus supporting cells. It is becoming increasingly evident that these supporting cells are recruited by cancer cells from nearby endogenous host stroma and promote events such as tumor angiogenesis, proliferation, invasion, and metastasis, as well as mediate mechanisms of therapeutic resistance. In addition, recruited stromal cells range in type and include vascular endothelial cells, pericytes, adipocytes, fibroblasts, and bone-marrow mesenchymal stromal cells. During normal wound healing and inflammatory processes, local stromal cells change their phenotype to become that of reactive stroma. Under certain conditions, however, tumor cells can co-opt these reactive stromal cells and further transition them into tumor-associated stromal cells (TASCs). These TASCs express higher levels of proteins, including alpha-smooth muscle actin, fibroblast activating protein, and matrix metalloproteinases, compared with their normal, non-reactive counterparts. TASCs are also known to secrete many pro-tumorigenic factors, including IL-6, IL-8, stromal-derived factor-1 alpha, vascular endothelial growth factor, tenascin-C, and matrix metalloproteinases, among others, which recruit additional tumor and pro-tumorigenic cells to the developing microenvironment. Here, we review the current literature pertaining to the origins of recruited host stroma, contributions toward tumor progression, tumor-associated stromal cells, and mechanisms of crosstalk between endogenous host stroma and tumor cells. -
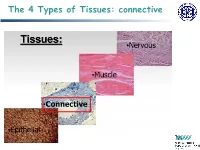
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

Índice De Denominacións Españolas
VOCABULARIO Índice de denominacións españolas 255 VOCABULARIO 256 VOCABULARIO agente tensioactivo pulmonar, 2441 A agranulocito, 32 abaxial, 3 agujero aórtico, 1317 abertura pupilar, 6 agujero de la vena cava, 1178 abierto de atrás, 4 agujero dental inferior, 1179 abierto de delante, 5 agujero magno, 1182 ablación, 1717 agujero mandibular, 1179 abomaso, 7 agujero mentoniano, 1180 acetábulo, 10 agujero obturado, 1181 ácido biliar, 11 agujero occipital, 1182 ácido desoxirribonucleico, 12 agujero oval, 1183 ácido desoxirribonucleico agujero sacro, 1184 nucleosómico, 28 agujero vertebral, 1185 ácido nucleico, 13 aire, 1560 ácido ribonucleico, 14 ala, 1 ácido ribonucleico mensajero, 167 ala de la nariz, 2 ácido ribonucleico ribosómico, 168 alantoamnios, 33 acino hepático, 15 alantoides, 34 acorne, 16 albardado, 35 acostarse, 850 albugínea, 2574 acromático, 17 aldosterona, 36 acromatina, 18 almohadilla, 38 acromion, 19 almohadilla carpiana, 39 acrosoma, 20 almohadilla córnea, 40 ACTH, 1335 almohadilla dental, 41 actina, 21 almohadilla dentaria, 41 actina F, 22 almohadilla digital, 42 actina G, 23 almohadilla metacarpiana, 43 actitud, 24 almohadilla metatarsiana, 44 acueducto cerebral, 25 almohadilla tarsiana, 45 acueducto de Silvio, 25 alocórtex, 46 acueducto mesencefálico, 25 alto de cola, 2260 adamantoblasto, 59 altura a la punta de la espalda, 56 adenohipófisis, 26 altura anterior de la espalda, 56 ADH, 1336 altura del esternón, 47 adipocito, 27 altura del pecho, 48 ADN, 12 altura del tórax, 48 ADN nucleosómico, 28 alunarado, 49 ADNn, 28 -

Reticulum Cell Sarcoma of Lymph Node with Mixed Dendritic and Fibroblastic Features Dan Jones, M.D., Ph.D., Mitual Amin, M.D., Nelson G
Reticulum Cell Sarcoma of Lymph Node with Mixed Dendritic and Fibroblastic Features Dan Jones, M.D., Ph.D., Mitual Amin, M.D., Nelson G. Ordonez, M.D., Armand B. Glassman, M.D., Kimberly J. Hayes, B.S., L. Jeffrey Medeiros, M.D. Division of Pathology and Laboratory Medicine, University of Texas-M.D. Anderson Cancer Center, Houston, Texas Lymph nodes contain a heterogeneous population We report a case of clinically aggressive reticulum of stromal cells with reticular morphology. These cell sarcoma with mixed follicular dendritic cell include antigen-presenting follicular dendritic cells (FDC) and fibroblastic reticular cell (FRC) features. (FDC) within the lymphoid follicle; interdigitating Histologically, the tumor was confined to lymph reticular cells (IDCs) related to the myeloid/mono- nodes occurring as a multifocal epithelioid and cytic lineage, and interfollicular fibroblastic reticu- spindle cell proliferation with appreciable mitotic lar cells (FRCs) of mesenchymal origin (1–4). The rate and numerous admixed non-neoplastic B-cells. FDC population includes multiple immunopheno- Ultrastructural examination revealed elongated typically distinct subsets of stromal cells within the cells with prominent nucleoli, interdigitating cell lymphoid follicle and mantle zone that regulate processes and frequent desmosomes. These fea- distinct stages of B cell differentiation (5, 6). IDCs tures are typical of FDC sarcoma. However, immu- have a primary function in antigen presentation in nohistochemical stains showed no expression of an- the interfollicular zones of the node and differenti- tigens characteristic of FDCs, including CD21, CD23 ate from Langerhans cells migrating from skin or and CD35. Cytogenetic characterization of this tu- from bone marrow-derived precursors (2).