In-Depth Characterization of Stromal Cells Within the Tumor Microenvironment Yields Novel Therapeutic Targets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Visualizing B Cell Development: Creating an Immunology Video Game
VISUALIZING B CELL DEVELOPMENT: CREATING AN IMMUNOLOGY VIDEO GAME By Emily Lunhui Ling A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Arts Baltimore, Maryland March, 2016 © 2016 Emily L. Ling All Rights Reserved ABSTRACT e foundational immunology concepts of lymphocyte development are important for beginning science students to comprehend. Video games oer the potential for a novel approach to teaching this complex subject matter by more eectively engaging students in this material. However, currently available educational video games intended to teach immunology have distinct limitations such as a lack of explicit demonstrations of the stages of lymphocyte development and clonal selection. is project identies the content focus and gameplay mechanics of currently available immunology video games. Using this as a basis, a novel approach for developing an immunology video game was outlined with the primary goal of improving integration of educational content. A proof of concept was developed for the B lymphocyte development portion of the game content and a partial prototype was developed in Unity 5 3D. e important contribution of this thesis was the development of a new approach to designing a more eective educational video game specically for immunology. Outcomes of this research will serve to inform future biomedical communicators on how to develop content for active learning games in immunology and provide a guide for designing full length educational video games featuring novel gameplay mechanics such as those identied through this project. Emily L. Ling ii CHAIRPERSONS OF THE SUPERVISORY COMMITTEE esis Preceptor Mark J. Soloski, Ph.D., Professor of Medicine Departments of Medicine, Pathology, Molecular Biology and Genetics, and Molecular Microbiology and Immunology Director, Immunology Training Program e Johns Hopkins University School of Medicine Departmental Advisor David A. -

Review Article Mesenchymal Stromal Cells Affect Disease Outcomes Via Macrophage Polarization
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref Hindawi Publishing Corporation Stem Cells International Volume 2015, Article ID 989473, 11 pages http://dx.doi.org/10.1155/2015/989473 Review Article Mesenchymal Stromal Cells Affect Disease Outcomes via Macrophage Polarization Guoping Zheng,1 Menghua Ge,1 Guanguan Qiu,1 Qiang Shu,2 and Jianguo Xu1,3 1 Shaoxing Second Hospital, Shaoxing, Zhejiang 312000, China 2The Children’s Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang 310052, China 3The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang 310003, China Correspondence should be addressed to Jianguo Xu; [email protected] Received 18 May 2015; Accepted 30 June 2015 Academic Editor: Armand Keating Copyright © 2015 Guoping Zheng et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Mesenchymal stromal cells (MSCs) are multipotent and self-renewable cells that reside in almost all postnatal tissues. In recent years, many studies have reported the effect of MSCs on the innate and adaptive immune systems. MSCs regulate the proliferation, activation, and effector function of T lymphocytes, professional antigen presenting cells (dendritic cells, macrophages, and B lymphocytes), and NK cells via direct cell-to-cell contact or production of soluble factors including indoleamine 2,3-dioxygenase, prostaglandin E2, tumor necrosis factor- stimulated gene/protein 6, nitric oxide, and IL-10. MSCs are also able to reprogram macrophages from a proinflammatory M1 phenotype toward an anti-inflammatory M2 phenotype capable of regulating immune response. -

Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1
2912 Vol. 8, 2912–2923, September 2002 Clinical Cancer Research Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1 Jennifer A. Tuxhorn, Gustavo E. Ayala, Conclusions: The stromal microenvironment in human Megan J. Smith, Vincent C. Smith, prostate cancer is altered compared with normal stroma and Truong D. Dang, and David R. Rowley2 exhibits features of a wound repair stroma. Reactive stroma is composed of myofibroblasts and fibroblasts stimulated to Departments of Molecular and Cellular Biology [J. A. T., T. D. D., express extracellular matrix components. Reactive stroma D. R. R.] and Pathology [G. E. A., M. J. S., V. C. S.] Baylor College of Medicine, Houston, Texas 77030 appears to be initiated during PIN and evolve with cancer progression to effectively displace the normal fibromuscular stroma. These studies and others suggest that TGF-1isa ABSTRACT candidate regulator of reactive stroma during prostate can- Purpose: Generation of a reactive stroma environment cer progression. occurs in many human cancers and is likely to promote tumorigenesis. However, reactive stroma in human prostate INTRODUCTION cancer has not been defined. We examined stromal cell Activation of the host stromal microenvironment is pre- phenotype and expression of extracellular matrix compo- dicted to be a critical step in adenocarcinoma growth and nents in an effort to define the reactive stroma environ- progression (1–5). Several human cancers have been shown to ment and to determine its ontogeny during prostate cancer induce a stromal reaction or desmoplasia as a component of progression. carcinoma progression. However, the specific mechanisms of Experimental Design: Normal prostate, prostatic intra- stromal cell activation are not known, and the extent to which epithelial neoplasia (PIN), and prostate cancer were exam- stroma regulates the biology of tumorigenesis is not fully un- ined by immunohistochemistry. -

Type 3 Innate Lymphoid Cells a Stromal Cell Niche for Human and Mouse
A Stromal Cell Niche for Human and Mouse Type 3 Innate Lymphoid Cells Kerim Hoorweg, Priyanka Narang, Zhi Li, Anne Thuery, Natalie Papazian, David R. Withers, Mark C. Coles and Tom This information is current as Cupedo of September 28, 2021. J Immunol 2015; 195:4257-4263; Prepublished online 16 September 2015; doi: 10.4049/jimmunol.1402584 http://www.jimmunol.org/content/195/9/4257 Downloaded from References This article cites 42 articles, 9 of which you can access for free at: http://www.jimmunol.org/content/195/9/4257.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2015 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology A Stromal Cell Niche for Human and Mouse Type 3 Innate Lymphoid Cells Kerim Hoorweg,*,1 Priyanka Narang,†,1 Zhi Li,† Anne Thuery,† Natalie Papazian,* David R. -

Tumor Microenvironment Consortium
Tumor Microenvironment Network (TMEN) Dinah Singer, Ph.D. Director Suresh Mohla, Ph.D. TMEN Program Director Division of Cancer Biology TMEN 2006-2011: Goals – Generate a comprehensive understanding of the composition of the normal stroma and the role of the stroma in tumor initiation, progression and metastasis – Develop resources and infrastructure critical to the broader research community to advance understanding of the tumor microenvironment (TME) TMEN 2006-2011: Current Program •Nine interdisciplinary groups characterizing the TME in major cancer sites, emphasizing human samples •Identifying and translating promising leads •Collaboratively addressing the complexity of TME by assessing: • The co-evolution of tumor-associated stromal cell types and the tumor •Stromal cell interactions with each other and with tumor cells •Generating novel reagents, models and technologies for the research community TMEN 2006-2011: Areas of Emphasis Microenvironment Initiation Progression Metastasis – Tumor initiating cells – Tumor heterogeneity – Stromal cell types and cellular processes – Complex signaling pathways – Genes and genetics TMEN 2006-2011: Scientific Accomplishments Tumor Initiating Cells: • Combined invasive gene signatures in tumor initiating cells and stromal wound repair response genes robustly predict metastasis-free and overall survival in breast, medulloblastoma, lung and prostate cancer patients • New therapeutic approaches to circumvent the chemo- and radio-resistance of tumor initiating cells Stromal Cells: • Delineation of mechanisms -

Massachusetts Licensed Motor Vehicle Damage Appraisers - Individuals September 05, 2021
COMMONWEALTH OF MASSACHUSETTS DIVISION OF INSURANCE PRODUCER LICENSING 1000 Washington Street, Suite 810 Boston, MA 02118-6200 FAX (617) 753-6883 http://www.mass.gov/doi Massachusetts Licensed Motor Vehicle Damage Appraisers - Individuals September 05, 2021 License # Licensure Individual Address City State Zip Phone # 1 007408 01/01/1977 Abate, Andrew Suffolk AutoBody, Inc., 25 Merchants Dr #3 Walpole MA 02081 0-- 0 2 014260 11/24/2003 Abdelaziz, Ilaj 20 Vine Street Lexington MA 02420 0-- 0 3 013836 10/31/2001 Abkarian, Khatchik H. Accurate Collision, 36 Mystic Street Everett MA 02149 0-- 0 4 016443 04/11/2017 Abouelfadl, Mohamed N Progressive Insurance, 2200 Hartford Ave Johnston RI 02919 0-- 0 5 016337 08/17/2016 Accolla, Kevin 109 Sagamore Ave Chelsea MA 02150 0-- 0 6 010790 10/06/1987 Acloque, Evans P Liberty Mutual Ins Co, 50 Derby St Hingham MA 02018 0-- 0 7 017053 06/01/2021 Acres, Jessica A 0-- 0 8 009557 03/01/1982 Adam, Robert W 0-- 0 West 9 005074 03/01/1973 Adamczyk, Stanley J Western Mass Collision, 62 Baldwin Street Box 401 MA 01089 0-- 0 Springfield 10 013824 07/31/2001 Adams, Arleen 0-- 0 11 014080 11/26/2002 Adams, Derek R Junior's Auto Body, 11 Goodhue Street Salem MA 01970 0-- 0 12 016992 12/28/2020 Adams, Evan C Esurance, 31 Peach Farm Road East Hampton CT 06424 0-- 0 13 006575 03/01/1975 Adams, Gary P c/o Adams Auto, 516 Boston Turnpike Shrewsbury MA 01545 0-- 0 14 013105 05/27/1997 Adams, Jeffrey R Rodman Ford Coll Ctr, Route 1 Foxboro MA 00000 0-- 0 15 016531 11/21/2017 Adams, Philip Plymouth Rock Assurance, 901 Franklin Ave Garden City NY 11530 0-- 0 16 015746 04/25/2013 Adams, Robert Andrew Country Collision, 20 Myricks St Berkley MA 02779 0-- 0 17 013823 07/31/2001 Adams, Rymer E 0-- 0 18 013999 07/30/2002 Addesa, Carmen E Arbella Insurance, 1100 Crown Colony Drive Quincy MA 02169 0-- 0 19 014971 03/04/2008 Addis, Andrew R Progressive Insurance, 300 Unicorn Park Drive 4th Flr Woburn MA 01801 0-- 0 20 013761 05/10/2001 Adie, Scott L. -

Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates
Research Article Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates David Gius,1 Margo C. Funk,2 Eric Y. Chuang,1 Sheng Feng,3 Phyllis C. Huettner,4 Loan Nguyen,2 C. Matthew Bradbury,1 Mark Mishra,1 Shuping Gao,1 Barbara M. Buttin,2 David E. Cohn,2 Matthew A. Powell,2 Neil S. Horowitz,2 Bradford P. Whitcomb,2 and JanetS. Rader 2 1Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Maryland and 2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology; 3Division of Biostatistics; and 4Lauren V. Ackerman Laboratory of Surgical Pathology, Washington University School of Medicine, St. Louis, Missouri Abstract (CIN 1–3) and finally to squamous cell carcinoma antigen (SCCA) This study is the first comprehensive, integrated approach to have been well characterized. Histologically, CIN 1 consists of examine grade-specific changes in gene expression along the immature basal-type cells involving the lower third of the entire neoplastic spectrum of cervical intraepithelial neoplasia epithelium. In CIN 2, these immature basal-type cells involve more (CIN) in the process of cervical carcinogenesis. This was than the lower third, whereas CIN 3 involves the full thickness of the accomplished by identifying gene expression signatures of epithelium. In addition, higher CIN grades exhibit nuclear crowding, disease progression using cDNA microarrays to analyze RNA pleomorphism, loss of cell polarity, and increased mitotic activity from laser-captured microdissected epithelium and underlying (1). These transitions seemto be well conserved and, as such, stroma from normal cervix, graded CINs, cancer, and patient- provide an intriguing systemto use genomicsto identify the early matched normal cervical tissues. -
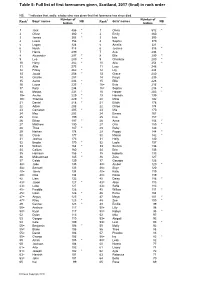
Table 5: Full List of First Forenames Given, Scotland, 2016 (Final) in Rank Order
Table 5: Full list of first forenames given, Scotland, 2017 (final) in rank order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Rank1 Boys' names NB Rank1 Girls' names NB babies babies 1 Jack 486 * 1 Olivia 512 * 2 Oliver 380 * 2 Emily 460 3 James 368 * 3 Isla 395 4 Lewis 356 4 Sophie 370 5 Logan 324 5 Amelia 321 6 Noah 318 6 Jessica 318 * 7 Harris 299 7 Ava 294 8 Alexander 297 * 8 Ella 290 * 9 Leo 289 * 9 Charlotte 280 * 10 Harry 282 * 10 Aria 254 * 11 Alfie 275 11 Lucy 248 12 Finlay 262 * 12 Lily 244 13 Jacob 258 * 13 Grace 240 14 Charlie 257 14 Freya 235 15 Aaron 236 * 15 Ellie 228 16 Lucas 235 * 16= Evie 216 17 Rory 234 16= Sophia 216 * 18 Mason 231 * 18 Harper 203 * 19= Archie 229 * 19 Hannah 199 19= Thomas 229 * 20 Millie 192 21 Daniel 218 * 21 Eilidh 178 22 Adam 208 22 Chloe 174 23 Cameron 205 * 23 Mia 170 24 Max 203 24 Emma 167 25 Finn 199 25 Eva 157 26 Ethan 197 * 26 Anna 154 * 27 Matthew 190 27 Orla 150 * 28 Theo 187 * 28 Ruby 146 29 Nathan 178 29 Poppy 144 * 30 Oscar 177 30 Maisie 142 * 31 Joshua 173 31 Holly 140 32 Brodie 170 * 32 Layla 137 33 William 164 * 33 Sienna 136 34 Callum 160 34 Erin 135 35 Harrison 156 * 35 Isabella 133 36 Muhammad 145 * 36 Zara 127 37 Caleb 139 37 Georgia 126 38= Jude 135 38= Amber 120 * 38= Samuel 135 38= Skye 120 40= Jamie 134 40= Katie 119 40= Ollie 134 40= Rosie 119 42 Liam 132 42 Daisy 116 * 43= Jaxon 127 * 43= Alice 113 43= Luke 127 43= Sofia 113 45= Freddie 126 45 Willow 111 * 45= Isaac 126 * 46 Esme 104 47= Angus 125 47 Maya 101 -

Colposcopy of the Uterine Cervix
THE CERVIX: Colposcopy of the Uterine Cervix • I. Introduction • V. Invasive Cancer of the Cervix • II. Anatomy of the Uterine Cervix • VI. Colposcopy • III. Histology of the Normal Cervix • VII: Cervical Cancer Screening and Colposcopy During Pregnancy • IV. Premalignant Lesions of the Cervix The material that follows was developed by the 2002-04 ASCCP Section on the Cervix for use by physicians and healthcare providers. Special thanks to Section members: Edward J. Mayeaux, Jr, MD, Co-Chair Claudia Werner, MD, Co-Chair Raheela Ashfaq, MD Deborah Bartholomew, MD Lisa Flowers, MD Francisco Garcia, MD, MPH Luis Padilla, MD Diane Solomon, MD Dennis O'Connor, MD Please use this material freely. This material is an educational resource and as such does not define a standard of care, nor is intended to dictate an exclusive course of treatment or procedure to be followed. It presents methods and techniques of clinical practice that are acceptable and used by recognized authorities, for consideration by licensed physicians and healthcare providers to incorporate into their practice. Variations of practice, taking into account the needs of the individual patient, resources, and limitation unique to the institution or type of practice, may be appropriate. I. AN INTRODUCTION TO THE NORMAL CERVIX, NEOPLASIA, AND COLPOSCOPY The uterine cervix presents a unique opportunity to clinicians in that it is physically and visually accessible for evaluation. It demonstrates a well-described spectrum of histological and colposcopic findings from health to premalignancy to invasive cancer. Since nearly all cervical neoplasia occurs in the presence of human papillomavirus infection, the cervix provides the best-defined model of virus-mediated carcinogenesis in humans to date. -

Lymphatic Tissue Engineering and Regeneration Laura Alderfer1, Alicia Wei1 and Donny Hanjaya-Putra1,2,3,4,5,6*
Alderfer et al. Journal of Biological Engineering (2018) 12:32 https://doi.org/10.1186/s13036-018-0122-7 REVIEW Open Access Lymphatic Tissue Engineering and Regeneration Laura Alderfer1, Alicia Wei1 and Donny Hanjaya-Putra1,2,3,4,5,6* Abstract The lymphatic system is a major circulatory system within the body, responsible for the transport of interstitial fluid, waste products, immune cells, and proteins. Compared to other physiological systems, the molecular mechanisms and underlying disease pathology largely remain to be understood which has hindered advancements in therapeutic options for lymphatic disorders. Dysfunction of the lymphatic system is associated with a wide range of disease phenotypes and has also been speculated as a route to rescue healthy phenotypes in areas including cardiovascular disease, metabolic syndrome, and neurological conditions. This review will discuss lymphatic system functions and structure, cell sources for regenerating lymphatic vessels, current approaches for engineering lymphatic vessels, and specific therapeutic areas that would benefit from advances in lymphatic tissue engineering and regeneration. Keywords: Lymphangiogenesis, Tissue Engineering, Disease Modeling, Wound Healing, Lymphedema, Stem Cells, Biomaterials, Interstitial Fluid, Regeneration I. Introduction to the Lymphatic System and its role Interstitial fluid (IF) is a plasma filtrate that is generated Function by transcapillary filtration and is governed by Starling The lymphatic system is nearly ubiquitous in the human forces, the net difference between hydrostatic and body, present in all tissues except the epidermis, cartil- osmotic pressures, at the microcirculatory level [9]. In age, eye lens, cornea, retina, and bone marrow [1, 2]. order to maintain fluid homeostasis, lymph formation in The main functions of the lymphatic system include the initial lymphatic vessels must be balanced by the net fluid homeostasis and interstitial fluid drainage, immune flux of plasma being filtered out [4]. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Are Mesenchymal Stromal Cells Immune Cells? Martin J Hoogduijn
Hoogduijn Arthritis Research & Therapy (2015) 17:88 DOI 10.1186/s13075-015-0596-3 REVIEW Open Access Are mesenchymal stromal cells immune cells? Martin J Hoogduijn however, covers various subsets of MSCs with different Abstract phenotypes and different functions [4,5]. Cell isolation Mesenchymal stromal cells (MSCs) are considered to be procedures can, therefore, affect the cellular compos- promising agents for the treatment of immunological ition of MSC cultures. Culture conditions can have a disease. Although originally identified as precursor cells further impact on the phenotype and function of MSCs for mesenchymal lineages, in vitro studies have [6]. This may affect study outcomes. Therefore, some demonstrated that MSCs possess diverse immune care should be taken in comparing the results of studies regulatory capacities. Pre-clinical models have shown using different MSC isolation and culture procedures. beneficial effects of MSCs in multiple immunological In the bone marrow, MSCs have a supportive function diseases and a number of phase 1/2 clinical trials carried for the haematopoietic system and provide a niche for out so far have reported signs of immune modulation haematopoietic progenitor cells to mature. The presence after MSC infusion. These data indicate that MSCs play a of MSCs is not limited, however, to the bone marrow central role in the immune response. This raises the and in other tissues, such as adipose tissue, muscle and academic question whether MSCs are immune cells multiple organs, they provide support for tissue cells by or whether they are tissue precursor cells with producing growth factors and matrix proteins. In immunoregulatory capacity. Correct understanding addition to their differentiation and tissue supportive of the immunological properties and origin of MSCs functions, MSCs have a well-established immune modu- will aid in the appropriate and safe use of the latory function.