Reactive Stroma in Human Prostate Cancer: Induction of Myofibroblast Phenotype and Extracellular Matrix Remodeling1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skeletal Reorganization and Chromatin Accessibility During Re- Vascularization-Based Blastema Regeneration
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 23 April 2021 doi:10.20944/preprints202104.0642.v1 Article Keratin5-BMP4 mechanosignaling network regulates cell cyto- skeletal reorganization and chromatin accessibility during re- vascularization-based blastema regeneration Xuelong Wang 1,2,4,†, Huiping Guo 1,6,†, Feifei Yu 1,†, Hui Zhang 1, Ying Peng 1, Chenghui Wang 3, Gang Wei 2, Jizhou Yan 1,5,* 1 Department of Developmental Biology, Institute for Marine Biosystem and Neurosciences, Shanghai Ocean University, Shanghai, China. 2 CAS Key Laboratory of Computational Biology, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, China 3 Department of Aquaculture, Shanghai Ocean University, Shanghai, China. 4 Present address: Pancreatic Disease Center, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong Univer- sity, Shanghai, China. 5 CellGene Bioscience, Shanghai, China. * Correspondence: [email protected] † These authors contributed equally to this study. Abstract: Heart regeneration after myocardial infarction remains challenging in reconstruction of blood resupply system. Here, we find that in zebrafish heart after resection of the ventricular apex, the local myocardial cells and the clotted blood cells undergo cell remodeling process via cytoplas- mic exocytosis and nuclear reorganization within revascularization-based blastema. The regenera- tive processes are visualized by spatiotemporal expression of three blastema representative factors (alpha-SMA- which marks for fibrogenesis, Flk1for angiogenesis/hematopoiesis, and Pax3a for re- musculogensis),and two histone modifications (H3K9Ac and H3K9Me3 mark for chromatin re- modeling). Using the cultured zebrafish embryonic fibroblasts we identify blastema fraction com- ponents and show that Krt5 peptide could link cytoskeleton network and BMP4 signaling pathway to regulate the transcription and chromatin accessibility at the blastema representative genes and bmp4 genes. -

Evidence for the Nonmuscle Nature of The" Myofibroblast" of Granulation Tissue and Hypertropic Scar. an Immunofluorescence Study
American Journal of Pathology, Vol. 130, No. 2, February 1988 Copyright i American Association of Pathologists Evidencefor the Nonmuscle Nature ofthe "Myofibroblast" ofGranulation Tissue and Hypertropic Scar An Immunofluorescence Study ROBERT J. EDDY, BSc, JANE A. PETRO, MD, From the Department ofAnatomy, the Department ofSurgery, and JAMES J. TOMASEK, PhD Division ofPlastic and Reconstructive Surgery, and the Department of Orthopaedic Surgery, New York Medical College, Valhalla, New York Contraction is an important phenomenon in wound cell-matrix attachment in smooth muscle and non- repair and hypertrophic scarring. Studies indicate that muscle cells, respectively. Myofibroblasts can be iden- wound contraction involves a specialized cell known as tified by their intense staining of actin bundles with the myofibroblast, which has morphologic character- either anti-actin antibody or NBD-phallacidin. Myofi- istics of both smooth muscle and fibroblastic cells. In broblasts in all tissues stained for nonmuscle but not order to better characterize the myofibroblast, the au- smooth muscle myosin. In addition, nonmuscle myo- thors have examined its cytoskeleton and surrounding sin was localized as intracellular fibrils, which suggests extracellular matrix (ECM) in human burn granula- their similarity to stress fibers in cultured fibroblasts. tion tissue, human hypertrophic scar, and rat granula- The ECM around myofibroblasts stains intensely for tion tissue by indirect immunofluorescence. Primary fibronectin but lacks laminin, which suggests that a antibodies used in this study were directed against 1) true basal lamina is not present. The immunocytoche- smooth muscle myosin and 2) nonmuscle myosin, mical findings suggest that the myofibroblast is a spe- components ofthe cytoskeleton in smooth muscle and cialized nonmuscle type of cell, not a smooth muscle nonmuscle cells, respectively, and 3) laminin and 4) cell. -

Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates
Research Article Profiling Microdissected Epithelium and Stroma to Model Genomic Signatures for Cervical Carcinogenesis Accommodating for Covariates David Gius,1 Margo C. Funk,2 Eric Y. Chuang,1 Sheng Feng,3 Phyllis C. Huettner,4 Loan Nguyen,2 C. Matthew Bradbury,1 Mark Mishra,1 Shuping Gao,1 Barbara M. Buttin,2 David E. Cohn,2 Matthew A. Powell,2 Neil S. Horowitz,2 Bradford P. Whitcomb,2 and JanetS. Rader 2 1Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, Maryland and 2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology; 3Division of Biostatistics; and 4Lauren V. Ackerman Laboratory of Surgical Pathology, Washington University School of Medicine, St. Louis, Missouri Abstract (CIN 1–3) and finally to squamous cell carcinoma antigen (SCCA) This study is the first comprehensive, integrated approach to have been well characterized. Histologically, CIN 1 consists of examine grade-specific changes in gene expression along the immature basal-type cells involving the lower third of the entire neoplastic spectrum of cervical intraepithelial neoplasia epithelium. In CIN 2, these immature basal-type cells involve more (CIN) in the process of cervical carcinogenesis. This was than the lower third, whereas CIN 3 involves the full thickness of the accomplished by identifying gene expression signatures of epithelium. In addition, higher CIN grades exhibit nuclear crowding, disease progression using cDNA microarrays to analyze RNA pleomorphism, loss of cell polarity, and increased mitotic activity from laser-captured microdissected epithelium and underlying (1). These transitions seemto be well conserved and, as such, stroma from normal cervix, graded CINs, cancer, and patient- provide an intriguing systemto use genomicsto identify the early matched normal cervical tissues. -

Colposcopy of the Uterine Cervix
THE CERVIX: Colposcopy of the Uterine Cervix • I. Introduction • V. Invasive Cancer of the Cervix • II. Anatomy of the Uterine Cervix • VI. Colposcopy • III. Histology of the Normal Cervix • VII: Cervical Cancer Screening and Colposcopy During Pregnancy • IV. Premalignant Lesions of the Cervix The material that follows was developed by the 2002-04 ASCCP Section on the Cervix for use by physicians and healthcare providers. Special thanks to Section members: Edward J. Mayeaux, Jr, MD, Co-Chair Claudia Werner, MD, Co-Chair Raheela Ashfaq, MD Deborah Bartholomew, MD Lisa Flowers, MD Francisco Garcia, MD, MPH Luis Padilla, MD Diane Solomon, MD Dennis O'Connor, MD Please use this material freely. This material is an educational resource and as such does not define a standard of care, nor is intended to dictate an exclusive course of treatment or procedure to be followed. It presents methods and techniques of clinical practice that are acceptable and used by recognized authorities, for consideration by licensed physicians and healthcare providers to incorporate into their practice. Variations of practice, taking into account the needs of the individual patient, resources, and limitation unique to the institution or type of practice, may be appropriate. I. AN INTRODUCTION TO THE NORMAL CERVIX, NEOPLASIA, AND COLPOSCOPY The uterine cervix presents a unique opportunity to clinicians in that it is physically and visually accessible for evaluation. It demonstrates a well-described spectrum of histological and colposcopic findings from health to premalignancy to invasive cancer. Since nearly all cervical neoplasia occurs in the presence of human papillomavirus infection, the cervix provides the best-defined model of virus-mediated carcinogenesis in humans to date. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Myofibroblasts in Schistosomal Portal Fibrosis Of
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 94(1): 87-93, Jan./Feb. 1999 87 Myofibroblasts in Schistosomal Portal Fibrosis of Man + Zilton A Andrade/ , Sylviane Guerret*, André LM Fernandes Centro de Pesquisas Gonçalo Moniz-Fiocruz , Rua Valdemar Falcão 121, 40295-001 Salvador, BA, Brasil *Laboratoire Marcel Merieux, Avenue Tony Garnier, Lyon, France Myofibroblasts, cells with intermediate features between smooth muscle cells and fibroblasts, have been described as an important cellular component of schistosomal portal fibrosis. The origin, distribu- tion and fate of myofibroblasts were investigated by means of light, fluorescent, immunoenzymatic and ultrastructural techniques in wedge liver biopsies from 68 patients with the hepatosplenic form of schistosomiasis. Results demonstrated that the presence of myofibroblasts varied considerably from case to case and was always related to smooth muscle cell dispersion, which occurred around medium- sized damaged portal vein branches. By sequential observation of several cases, it was evident that myofibroblasts derived by differentiation of vascular smooth muscle and gradually tended to disappear, some of them further differentiating into fibroblasts. Thus, in schistosomal pipestem fibrosis myofibroblasts appear as transient cells, focally accumulated around damaged portal vein branches, and do not seem to have by themselves any important participation in the pathogenesis of hepatosplenic schistoso- miasis. Key words: myofibroblast - schistosomiasis - pipestem fibrosis - liver The characteristic -

Tumor-Associated Stromal Cells As Key Contributors to the Tumor Microenvironment Karen M
Bussard et al. Breast Cancer Research (2016) 18:84 DOI 10.1186/s13058-016-0740-2 REVIEW Open Access Tumor-associated stromal cells as key contributors to the tumor microenvironment Karen M. Bussard1,2, Lysette Mutkus3, Kristina Stumpf3, Candelaria Gomez-Manzano4 and Frank C. Marini1,3* Abstract The tumor microenvironment is a heterogeneous population of cells consisting of the tumor bulk plus supporting cells. It is becoming increasingly evident that these supporting cells are recruited by cancer cells from nearby endogenous host stroma and promote events such as tumor angiogenesis, proliferation, invasion, and metastasis, as well as mediate mechanisms of therapeutic resistance. In addition, recruited stromal cells range in type and include vascular endothelial cells, pericytes, adipocytes, fibroblasts, and bone-marrow mesenchymal stromal cells. During normal wound healing and inflammatory processes, local stromal cells change their phenotype to become that of reactive stroma. Under certain conditions, however, tumor cells can co-opt these reactive stromal cells and further transition them into tumor-associated stromal cells (TASCs). These TASCs express higher levels of proteins, including alpha-smooth muscle actin, fibroblast activating protein, and matrix metalloproteinases, compared with their normal, non-reactive counterparts. TASCs are also known to secrete many pro-tumorigenic factors, including IL-6, IL-8, stromal-derived factor-1 alpha, vascular endothelial growth factor, tenascin-C, and matrix metalloproteinases, among others, which recruit additional tumor and pro-tumorigenic cells to the developing microenvironment. Here, we review the current literature pertaining to the origins of recruited host stroma, contributions toward tumor progression, tumor-associated stromal cells, and mechanisms of crosstalk between endogenous host stroma and tumor cells. -
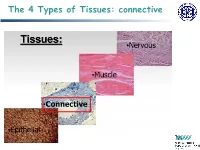
The 4 Types of Tissues: Connective
The 4 Types of Tissues: connective Connective Tissue General structure of CT cells are dispersed in a matrix matrix = a large amount of extracellular material produced by the CT cells and plays a major role in the functioning matrix component = ground substance often crisscrossed by protein fibers ground substance usually fluid, but it can also be mineralized and solid (bones) CTs = vast variety of forms, but typically 3 characteristic components: cells, large amounts of amorphous ground substance, and protein fibers. Connective Tissue GROUND SUBSTANCE In connective tissue, the ground substance is an amorphous gel-like substance surrounding the cells. In a tissue, cells are surrounded and supported by an extracellular matrix. Ground substance traditionally does not include fibers (collagen and elastic fibers), but does include all the other components of the extracellular matrix . The components of the ground substance vary depending on the tissue. Ground substance is primarily composed of water, glycosaminoglycans (most notably hyaluronan ), proteoglycans, and glycoproteins. Usually it is not visible on slides, because it is lost during the preparation process. Connective Tissue Functions of Connective Tissues Support and connect other tissues Protection (fibrous capsules and bones that protect delicate organs and, of course, the skeletal system). Transport of fluid, nutrients, waste, and chemical messengers is ensured by specialized fluid connective tissues, such as blood and lymph. Adipose cells store surplus energy in the form of fat and contribute to the thermal insulation of the body. Embryonic Connective Tissue All connective tissues derive from the mesodermal layer of the embryo . The first connective tissue to develop in the embryo is mesenchyme , the stem cell line from which all connective tissues are later derived. -

Development and Characterisation of 3D Skeletal Muscle Constructs Under Defined Mechanical Regulation
Development and Characterisation of 3D Skeletal Muscle Constructs under Defined Mechanical Regulation By Umber Cheema, B.Sc (Hons) UCL A thesis submitted in partial fulfilment for the Degree of Doctor of Philosophy Royal Free and University College Medical School July 2004 Department of Surgery, Charles Bell House, 64 Riding House Street, London W1W 7EJ. Department of Anatomy and Developmental Biology, Royal Free and University College Medical school, Royal Free Campus, Rowland Hill street, London NW2 2PF 1 UMI Number: U591876 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U591876 Published by ProQuest LLC 2013. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Acknowledgements I would like to thank my primary supervisor, Robert Brown, for all his help, encouragement, guidance and support over the course of my studies. He has helped make this whole learning experience a truly enjoyable one. I am also deeply thankful for the support of my second supervisor, Geoffrey Goldspink, who has given me a sound understanding of muscle and for giving me the opportunity to study. I would like to thank all the staff and students I have had the privilege of working with. -

Fibroblasts and Myofibroblasts in Wound Healing: Force Generation
Journal of Tissue Viability (2011) 20, 108e120 www.elsevier.com/locate/jtv Basic research Fibroblasts and myofibroblasts in wound healing: Force generation and measurement Bin Li a, James H.-C. Wang b,* a Orthopedic Institute, Soochow University, 708 Renmin Road, Suzhou, Jiangsu 215007, China b MechanoBiology Laboratory, Departments of Orthopaedic Surgery, Bioengineering, and Mechanical Engineering and Materials Science, University of Pittsburgh, 210 Lothrop St, BST E1640, Pittsburgh, PA 15213, USA KEYWORDS Abstract Fibroblasts are one of the most abundant cell types in connective Fibroblasts; tissues. These cells are responsible for tissue homeostasis under normal physiolog- Myofibroblasts; ical conditions. When tissues are injured, fibroblasts become activated and differ- Wounds; entiate into myofibroblasts, which generate large contractions and actively Contraction; produce extracellular matrix (ECM) proteins to facilitate wound closure. Both fibro- Measurement blasts and myofibroblasts play a critical role in wound healing by generating trac- tion and contractile forces, respectively, to enhance wound contraction. This review focuses on the mechanisms of force generation in fibroblasts and myofibro- blasts and techniques for measuring such cellular forces. Such a topic was chosen specifically because of the dual effects that fibroblasts/myofibroblasts have in wound healing processe a suitable amount of force generation and matrix deposi- tion is beneficial for wound healing; excessive force and matrix production, however, result in tissue scarring and even malfunction of repaired tissues. There- fore, understanding how forces are generated in these cells and knowing exactly how much force they produce may guide the development of optimal protocols for more effective treatment of tissue wounds in clinical settings. ª 2009 Tissue Viability Society. -

Enhanced Upregulation of Smooth Muscle Related Transcripts by Tgfb2 in Asthmatic (Myo) Fibroblasts J Wicks, H M Haitchi, S T Holgate, D E Davies, R M Powell
313 ASTHMA Thorax: first published as 10.1136/thx.2005.050005 on 31 January 2006. Downloaded from Enhanced upregulation of smooth muscle related transcripts by TGFb2 in asthmatic (myo) fibroblasts J Wicks, H M Haitchi, S T Holgate, D E Davies, R M Powell ............................................................................................................................... Thorax 2006;61:313–319. doi: 10.1136/thx.2005.050005 Background: Transforming growth factor beta (TGFb) upregulates a number of smooth muscle specific genes in (myo)fibroblasts. As asthma is characterised by an increase in airway smooth muscle, we postulated that TGFb2 favours differentiation of asthmatic (myo)fibroblasts towards a smooth muscle phenotype. Methods: Primary fibroblasts were grown from bronchial biopsy specimens from normal (n = 6) and asthmatic (n = 7) donors and treated with TGFb2 to induce myofibroblast differentiation. The most stable genes for normalisation were identified using RT-qPCR and the geNorm software applied to a panel of 12 See end of article for authors’ affiliations ‘‘housekeeping’’ genes. Expression of a-smooth muscle actin (aSMA), heavy chain myosin (HCM), ....................... calponin 1 (CPN 1), desmin, and c-actin were measured by RT-qPCR. Protein expression was assessed by immunocytochemistry and western blotting. Correspondence to: Dr R M Powell, The Brooke Results: Phospholipase A2 and ubiquitin C were identified as the most stably expressed and practically Laboratory, Level F, South useful genes for normalisation of gene expression during myofibroblast differentiation. TGFb2 induced Block, Southampton mRNA expression for all five smooth muscle related transcripts; aSMA, HCM and CPN 1 protein were also General Hospital, Southampton SO16 6YD, increased but desmin protein was not detectable. Although there was no difference in basal expression, UK; [email protected] HCM, CPN 1 and desmin were induced to a significantly greater extent in asthmatic fibroblasts than in those from normal controls (p = 0.041 and 0.011, respectively). -

Interaction of Stellate Cells with Pancreatic Carcinoma Cells
Cancers 2010, 2, 1661-1682; doi:10.3390/cancers2031661 OPEN ACCESS cancers ISSN 2072-6694 www.mdpi.com/journal/cancers Review Interaction of Stellate Cells with Pancreatic Carcinoma Cells Hansjörg Habisch 1, Shaoxia Zhou 1, Marco Siech 2 and Max G. Bachem 1,* 1 Department Clinical Chemistry and Central Laboratory, University of Ulm, Germany; E-Mails: [email protected] (H.H); [email protected] (S.Z.) 2 Department of surgery, Ostalbklinikum Aalen, Germany; E-Mail: [email protected] (M.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-731-500-67500/01; Fax: +49-731-500-67506. Received: 28 July 2010; in revised form: 2 September 2010 / Accepted: 2 September 2010 / Published: 9 September 2010 Abstract: Pancreatic cancer is characterized by its late detection, aggressive growth, intense infiltration into adjacent tissue, early metastasis, resistance to chemo- and radiotherapy and a strong ―desmoplastic reaction‖. The dense stroma surrounding carcinoma cells is composed of fibroblasts, activated stellate cells (myofibroblast-like cells), various inflammatory cells, proliferating vascular structures, collagens and fibronectin. In particular the cellular components of the stroma produce the tumor microenvironment, which plays a critical role in tumor growth, invasion, spreading, metastasis, angiogenesis, inhibition of anoikis, and chemoresistance. Fibroblasts, myofibroblasts and activated stellate cells produce the extracellular matrix components and are thought to interact actively with tumor cells, thereby promoting cancer progression. In this review, we discuss our current understanding of the role of pancreatic stellate cells (PSC) in the desmoplastic response of pancreas cancer and the effects of PSC on tumor progression, metastasis and drug resistance.