FAO Fisheries & Aquaculture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Field Identification Guide to the Living Marine Resources In
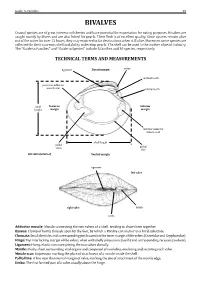
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Proceedings of the Academy of Natural Sciences of Philadelphia
32 PROCEEDINGS OF THE ACADEMY OF [1887. ON NEW GENEEIC FOKMS OF CRETACEOUS MOLLUSCA AND THEIE RELATION TO OTHER FORMS. BY CHARLES A. WHITE. Published by permission of the Director of the United States Geological Survey. The type species of the three generic forms which are described in this article ^ belong to the collections of Cretaceous fossils from Texas, which I am now preparing for publication in one of the memoirs of the U. S. Geological Survey. In their generic charac- teristics all three of them appear to be respectively identical with certain forms which have long been known, but which have been referred to other genera by different authors. The features which I now present as having generic value seem to have been overlooked by those authors, or, so far as they were observed, they were treated as specific characters. Two of these forms belong to the section Melininse of the family Aviculidse. The other is referred to the Crassatellidse, but it departs considerably from the typical section of that family. CRASSATELLIDJE. Genus STEARNSIA (gen. nov.). Shell compressed, subtrihedral or subcircular in marginal out- line; beaks small, closely approximate, prominent by reason of the abrupt sloping away of both the antero-and postero-dorsal borders; lunule and escutcheon both well defined and flattened or excavated; hinge strong, consisting of both cardinal and lateral teeth; cardinal teeth two in the left valve and three in the right; both posterior and anterior lateral teeth long and slender; posterior laterals two in the right valve and one in the left; anterior laterals two in the left valve and one in the right. -

Three Alien Molluscs from Iskenderun Bay (SE Turkey)
Aquatic Invasions (2006) Volume 1, Issue 2: 76-79 DOI 10.3391/ai.2006.1.2.4 © 2006 The Author(s) Journal compilation © 2006 REABIC (http://www.reabic.net) This is an Open Access article Research article Three alien molluscs from Iskenderun Bay (SE Turkey) Doğan Çeviker1 and Serhat Albayrak2* 1Itri Sokak No:2 34349 Balmumcu-Istanbul, Turkey E-mail: [email protected] 2Istanbul University, Faculty of Science, Department of Biology 34118 Vezneciler-Istanbul, Turkey E-mail: [email protected] *Corresponding author Received 26 April 2006; accepted in revised form 4 May 2006 Abstract This study reports the presence of three alien molluscs from Iskenderun Bay (SE Turkey). Amathina tricarinata (Linnaeus, 1767) and Petricola hemprichi Issel, 1869 have prior records from other regions of Mediterranean, but, Cardites akabana (Sturany, 1899) first recorded in this paper. Since all of them are present in the Red Sea or Suez Canal, they can be considered as Lessepsian immigrants. Key words: Mollusca, alien species, Mediterranean, Turkey Introduction that 88 % of the exotic molluscs are Lessepsian immigrants in the eastern Mediterranean (Galil The Mediterranean Sea hosts about 8500 species and Zenetos 2002). Detailed data about these species of macroscopic animals. This rich biodiversity, are available on the Internet (www.ciesm.org/atlas). representing 8-9 % of total species number of the Either Lessepsian or non-Lessepsian, many world’s seas, comprises temperate and sub- new non-indigenous species continue to enter the tropical elements together with endemic and Mediterranean. alien species (Zenetos et al. 2002). The eastern Mediterranean is most vulnerable The introduction of alien species (also known to invasion and should be continuously as exotic, introduced or non-native species) into monitored. -

Eoursivivas Cultriformis
Page 118 The Veliger, Vol. 47, No. 2 Figures 40-53. Specimens coated with ammonium chloride. Figures 40-45. Panzacorbula Squires & Saul, gen. nov. pozo (Dailey & Popenoe, 1966). Figure 40. Holotype LACMIP 8916, LACMIP loc. 23774, left valve, X2.2. Figure 41. Paratype LACMIP 8918, LACMIP loc. 23774, left-valve interior, X2.1. Figure 42. Paratype LACMIP 8917, LACMIP loc. 23774, right valve, X2.1. Figure 43. Hypotype LACMIP 13124, LACMIP loc. 10667, immature right valve, X4.1. Figure 44. Paratype LACMIP 8917, LACMIP loc. 23774, right-valve interior, X2. Figure 45. Holotype LACMIP 8916, LACMIP loc. 23774, dorsal view, X2. Figures 46-49. Eoursivivas cultri- formis (Gabb, 1864). Figure 46. Hypotype LACMIP 13125, LACMIP loc. 26345, left valve, X2.6. Figure 47. Hypotype LACMIP 13126, LACMIP loc. 26345, left valve, X5.1. Figure 48. Lectotype UCMP 11945a, CGS loc. 144, right valve, X5.2. Figure 49. Hypotype LACMIP 13127, LACMIP loc. 26345, right valve, X2.3. Figures 50-53. Caestocorbula cavus Squires & Saul, sp. nov., UCMP loc. B- 5611. Figure 50. Paratype UCMP 155540, left valve, XI 3.7. Figures 51-53. Holotype UCMP 155539, X7. Figure 51. Left valve. Figure 52. Right valve. Figure 53. Dorsal view. R. L. Squires & L. R. Saul, 2004 Page 119 Diagnosis: Same as for genus. Discussion: This study of Dailey and Popenoe's species Description: Shell medium (maximum length 21.7 mm); is based on 124 specimens (including the type material): moderately thick. Valves subpyriform to trigonal elon- 96 right valves, 25 left valves, and three pairs of con- gate, inflated (right valve more inflated than left valve), joined valves. -

Guide to Estuarine and Inshore Bivalves of Virginia
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1968 Guide to Estuarine and Inshore Bivalves of Virginia Donna DeMoranville Turgeon College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Marine Biology Commons, and the Oceanography Commons Recommended Citation Turgeon, Donna DeMoranville, "Guide to Estuarine and Inshore Bivalves of Virginia" (1968). Dissertations, Theses, and Masters Projects. Paper 1539617402. https://dx.doi.org/doi:10.25773/v5-yph4-y570 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. GUIDE TO ESTUARINE AND INSHORE BIVALVES OF VIRGINIA A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts LIBRARY o f the VIRGINIA INSTITUTE Of MARINE. SCIENCE. By Donna DeMoranville Turgeon 1968 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts jfitw-f. /JJ'/ 4/7/A.J Donna DeMoranville Turgeon Approved, August 1968 Marvin L. Wass, Ph.D. P °tj - D . dvnd.AJlLJ*^' Jay D. Andrews, Ph.D. 'VL d. John L. Wood, Ph.D. William J. Hargi Kenneth L. Webb, Ph.D. ACKNOWLEDGEMENTS The author wishes to express sincere gratitude to her major professor, Dr. -

Chapter I Taxonomy
THE AMERICAN OYSTER CRASSOSTREA VIRGINICA GMELIN By PAUL S. GALTSOFF, Fishery Biologist BUREAU OF COMMERCIAL FISHERIES CHAPTER I TAXONOMY Page This broad characterization included a number Taxonomic characters _ 4 SheIL _ 4 of genera such as scallops, pen shells (Pinnidae), Anatomy _ 4 Sex and spawnlng _ limas (Limidae) and other mollusks which ob 4 Habitat _ 5 viously are not oysters. In the 10th edition of Larvll! shell (Prodlssoconch) _ 6 "Systema Naturae," Linnaeus (1758) wrote: The genera of living oysters _ 6 Genus 08trea _ 6 "Ostreae non orones, imprimis Pectines, ad Genus Cra8808trea _ 7 Genus Pycnodonte _ cardinem interne fulcis transversis numerosis 7 Bibliography _ 14 parallelis in utraque testa oppositis gaudentiquae probe distinguendae ab Areis polypleptoginglymis, The family Ostreidae consists of a large number cujus dentes numerosi alternatim intrant alterius of edibleand nonedible oysters. Their distribution sinus." Le., not all are oysters, in particular the is confined to a broad belt of coastal waters within scallops, which have many parallel ribs running the latitudes 64° N. and 44° S. With few excep crosswise inward toward the hinge on each shell tions oysters thrive in shallow water, their vertical on opposite sides; these should properly be dis distribution extending from a level approximately tinguished from Area polyleptoginglymis whose halfway between high and low tide levels to a many teeth alternately enter between the teeth depth of about 100 feet. Commercially exploited of the other side. oyster beds are rarely found below a depth of 40 In the same publication the European flat feet. oyster, Ostrea edulis, is described as follows: The· name "Ostrea" was given by Linnaeus "Vulgo Ostrea dictae edulis. -

The Earliest Fossil Record of the Poorly Known Family Condylocardiidae from Argentina
Andean Geology ISSN: 0718-7092 ISSN: 0718-7106 [email protected] Servicio Nacional de Geología y Minería Chile The earliest fossil record of the poorly known family Condylocardiidae from Argentina Pérez, Damián Eduardo The earliest fossil record of the poorly known family Condylocardiidae from Argentina Andean Geology, vol. 46, no. 2, 2019 Servicio Nacional de Geología y Minería, Chile Available in: https://www.redalyc.org/articulo.oa?id=173961655010 DOI: https://doi.org/10.5027/andgeoV46n2-3130 This work is licensed under Creative Commons Attribution 3.0 International. PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Damián Eduardo Pérez. The earliest fossil record of the poorly known family Condylocardiidae from... e earliest fossil record of the poorly known family Condylocardiidae from Argentina El registro fósil más antiguo de la poco conocida familia Condylocardiidae en Argentina. Damián Eduardo Pérez DOI: https://doi.org/10.5027/andgeoV46n2-3130 Museo Argentino de Ciencias Naturales Bernardino Redalyc: https://www.redalyc.org/articulo.oa? Rivadavia, Argentina id=173961655010 [email protected] Received: 07 December 2017 Accepted: 13 September 2018 Published: 04 February 2019 Abstract: e scarcely known family Condylocardiidae (Bivalvia: Archiheterodonta) is poorly represented in the fossil record and their living representatives are also poorly known. is work presents a new representative of the family from the early Pliocene of marine terrace of Cerro Laciar (Santa Cruz Province). Carditella pitufina sp. nov. is described and characterized by a shell large for the genus, 15 radial ribs as wide as interspaces, high hinge plate and broad and large hinge teeth. -

Calyptogena Diagonalis, a New Vesicomyid Bivalve from Subduction Zone Cold Seeps in the Eastern North Pacific JAMES P
THE VELIGER @ CMS, Inc., 1999 The Veliger 42(2):117-123 (April 1, 1999) Calyptogena diagonalis, a New Vesicomyid Bivalve from Subduction Zone Cold Seeps in the Eastern North Pacific JAMES P. BARRY Monterey Bay Aquarium Research Institute, PO. Box 628, Moss Landing, California 95039, USA AND RANDALLE.KOCHEVAR Monterey Bay Aquarium, Pacific Grove, California 93950, USA Abstract. A new vesicomyid bivalve species, Calyptogena diagonalis, is described from cold seep communities in the Cascadia subduction zone off the Oregon coast and accretionary wedge sediments along the Pacific coast of Costa Rica. Live bivalves and shells were collected at sulfide seeps near 2021 m depth in Oregon and from 2900 to 3800 m depth in Costa Rica. Shell morphology of C. diagonalis differs considerably from sympatric congeneric and confamilial species of the northeastern Pacific. Shells are large (to 24.0 cm) and elongate (H/L = 0.42), with one or more ridges on the external shell surface extending diagonally from the umbo to near the posteroventral margin. Enlarged, sulfur- colored ctenidia and micrographs of endosymbiotic bacteria held in ctenidia suggest that this species, like other vesi- comyids, is a sulfur-based chemolithoautotroph. INTRODUCTION derstanding of the natural history and biology of vesi- comyids, including description of many new species. Ear- The bivalve family Vesicomyidae, first established by ly trawl and dredge samplers were deployed most com- Dall & Simpson (1901) includes more than 50 species monly over soft sediments, thereby undersampling found nearly exclusively in sulfide-rich habitats such as geologically rugged terrain where seep and vent habitats cold seeps, hydrothermal vents, and accumulations of or- often occur. -

Proceedings of the United States National Museum
NEW MOLLUSCAN GENP]RA FROM THE CARBONIFEROUS." By Geouce H. Girty, Cuslodlaii of CdrhoiiiJ'croiis Innniehrutr Foi<hUs. Among- the Carboniferous faunas examined in the course of investi- gations connected with othcial work, 1 have been hnl to recognize a large numl)er of undescribed species and some genera, which in most cases it did not seem appropi'iate to make known in connection with the studies that brouglit them into notice. IShuiy of tliesc types were laid aside for discussion with one or another of a number of subjects the investigation of which is projected. Thei'e remains, how- ever, a collection very miscellaneous in character and not germane to any of the papers now in view. A few of the generic types it is here proposed to describe and name. In order to secure brevity in the title of this paper, the term raol- luscan is employed in a somewhat broader sense than present jusage generally saiK'tions, though not inconsistently with that of the last gen- eration by which brachiopoda were grouped with the true mollusca. The fossils upon which the observations recorded in this paper were made form part of the collections of the U. S. National Museum. LIMIPECTEN,'' new genus. It is rare that one is able directly to ol)serve structural characters in Carboniferous Pectinoids. Usually either the shell is embedded in hard rock, from which it is hopeless to clear It, or else, and this is the best that happens, the test has been dissolved away and the structures are seen in reverse as casts. -

<I>Fimbria Fimbriata</I>
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Morton, Brian, 1979. The biology and functional morphology of the coral- sand bivalve Fimbria fimbriata (Linnaeus 1758). Records of the Australian Museum 32(11): 389–420, including Malacological Workshop map. [31 December 1979]. doi:10.3853/j.0067-1975.32.1979.468 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia 389 THE BIOLOGY AND FUNCTIONAL MORPHOLOGY OF THE CORAL-SAND BIVALVE FIMBRIA FIMBRIATA (Linnaeus 1758). BY BRIAN MORTON Department of Zoology, The University of Hong Kong SUMMARY Fimbria fimbriata Linnaeus 1758 is an infaunal inhabitant of coral sands in the Indo-Pacific. The structure and mineralogy of the shell (Taylor, Kennedy and Hall, 1973) confirms its taxonomic position as a member of the Lucinacea. Nicol (1950) erected (giving no reasons) a new family, taking its name (the Fimbriidae) from the genus. This study supports the view of Alien and Turner (1970) and Boss (1970) that Fimbria is closely related to the Lucinidae Fleming 1828 though a study of fossil fimbriids will have to be undertaken before the extreme view of Alien and Turner (1970) that Fimbria is a lucinid, can be validated. The Lucinidae and F. fimbriata possess the following features in common. 1. An enlarged anterior half of the shell with an antero-dorsal inhalant stream. 2. A single (inner) demibranch with type G ciliation (Atkins, 1937b). -

Bulletin of the United States Fish Commission Seattlenwf
NATURAL HISTORY AND PROPAGATION OF FRESH-WATER MUSSELS $ By R. E. Coker, Assistant in Charge Scientific Inquiry A. F. Shira, Lately Director Fisheries Biological Station, Fairport, Iowa H. W. Clark, Scientific Assistant, and A. D. Howard, Scientific Assistant 75 Blank page retained for pagination CONTENTS • .;f. Page. Introduction. ............................................................................. 79 Part I. Natural history of fresh-water mussels. .............................................. 81 Habits................................................................................. 81 Conditions of existence :........................ 81 Locomotion. ...................................................................... 82 Density of population. .. .. .... ...................................................... 84 Breeding. .......................................................................... 85 Winterhabits ,......... 85 Feeding habits , ....................................... 86 Food of mussels ".......................................... 87 Significance of the problem. ....................................................... 87 Observations of Franz Schrader on food of mussels. .. .. .. .. .. ............... 88 Species studied "........................... .. ....... 88 Food content of waters '" 88 Food discrimination under normal conditions. .. .. .. ... 89 Utilization of food materials. .. .. .. .. .. .. ......... 89 Experiments in feeding vegetable matter. ....................................... 89 Experiments in feeding -

Bivalvia 101
Bivalvia 101 An Introduction to Marine Bivalves Paul ValentichValentich--ScottScott Curator of Malacology Santa Barbara Museum of Natural History Beautiful Bivalves of Southern California Class Bivalvia = two valves (shells) Clams,,,p,y mussels, scallops, oysters and their kin • Two part shell Bivalvia Clams, mussels, scallops, oysters and their kin • Two part shell • No head Bivalvia Clams, mussels, scallops, oysters and their kin • Two part shell • No head • Most are filter feeders Bivalvia Clams, mussels, scallops, oysters and their kin • Two part shell • No head • Most are filter feeders • Broadcast spawners Bivalvia Clams, mussels, scallops, oysters and their kin • Bivalves are great to EAT!!! Bivalve Orienteering Lookinggp from the top – Dorsal view Bival ve O ri ent eeri ng Posterior Anterior How do we know which end is which?? Bival ve O ri ent eeri ng Ligament Posterior Anterior Liggypament is usually posterior Bival ve O ri ent eeri ng BkBeaks Posterior Anterior Beaks often point anterior Bival ve O ri ent eeri ng Left valve Posterior Anterior Right valve Determining left from right Bival ve O ri ent eeri ng Left hand Left valve Anterior pointed away from you Right hand Right valve Anterior pointed Quick hint away from you Bivalve Orienteering Dorsal Posterior Anterior Ventral Looking from the side – Lateral view Bivalve Orienteering Dorsal Posterior Anterior Ventral Looking on the inside Bivalve Characters Umbo Beak Beak vs. Umbo Bivalve Characters –Sculpture– Sculpture Radial Sculpture Bivalve Characters –Sculpture– Sculpture