Calyptogena Packardana, a New Species of Vesicomyid Bivalve from Cold Seeps in Monterey Bay, California By
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Field Identification Guide to the Living Marine Resources In
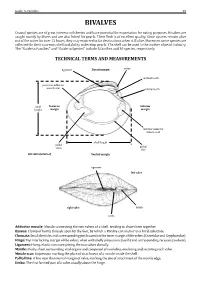
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Female Description of the Hydrothermal Vent Cephalopod Vulcanoctopus Hydrothermalis A.F
Journal of the Marine Biological Association of the United Kingdom, 2008, 88(2), 375–379. #2008 Marine Biological Association of the United Kingdom doi:10.1017/S0025315408000647 Printed in the United Kingdom Female description of the hydrothermal vent cephalopod Vulcanoctopus hydrothermalis a.f. gonzÆlez1, a. guerra1, s. pascual1 and m. segonzac2 1ECOBIOMAR, Instituto de Investigaciones Marinas (CSIC), Eduardo Cabello 6, 36208 Vigo, Spain, 2IFREMER, Centre de Brest, Laboratoire Environnement Profond, BP 70, 29280-Plouzane´, France During biological sampling of hydrothermal vents on the East Pacific Rise, the manned submersible ‘Nautile’ caught the first female of the endemic cephalopod Vulcanoctopus hydrothermalis. The specimen caught at the vent site Gromit (21833 660S, 114817 980W at 2832 m depth) is described here in detail and an amended diagnosis of the species proposed. The external morphology, measurements and internal structure resemble that of males of this species. One of the most remarkable char- acters is the lack of spermathecae and the absence of apical filaments in the oocytes to provide a site for sperm storage. It is suggested that some species of the genera Benthoctopus and Bathypolypus would be the most suitable octopod ancestor of V. hydrothermalis. Keywords: hydrothermal vent, cephalopods, Vulcanoctopus hydrothermalis, female description Submitted 20 April 2007; accepted 29 November 2007 INTRODUCTION et al., 2006). It inhabits an isolated extreme environment very close to the base of the chimneys and is also observed The study of chemosynthetic ecosystems in the deep sea rep- on the pillow lava at several metres from the active areas. resents a challenging issue due to the difficulty of sampling, This benthic species has characters that represent adaptations which involves the use of modern technologies such as either to the deep-sea or to a hydrothermal vent habitat manned submersibles. -

FAO Fisheries & Aquaculture
Food and Agriculture Organization of the United Nations Fisheries and for a world without hunger Aquaculture Department Cultured Aquatic Species Information Programme Mercenaria mercenaria (Linnaeus, 1758) I. Identity V. Status And Trends a. Biological Features VI. Main Issues b. Images Gallery a. Responsible Aquaculture Practices II. Profile VII. References a. Historical Background a. Related Links b. Main Producer Countries c. Habitat And Biology III. Production a. Production Cycle b. Production Systems c. Diseases And Control Measures IV. Statistics a. Production Statistics b. Market And Trade Identity Mercenaria mercenaria Linnaeus, 1758 [Veneridae] FAO Names: En - Northern quahog(=Hard clam), Fr - Praire, Es - Chirla mercenaria Biological features Shell solid, equivalve; inequilateral, beaks in the front half of the shell; broadly oval in outline. Ligament a deeply inset, dark brown elliptical band, behind the beaks reaching half-way to the posterior margin. Lunule well defined, broad, heart-shaped. Escutcheon indistinct. Sculpture of concentric lines, raised here and there into ridges, and fine radiating lines. In young specimens the ridges are present all over the shell but in the adult they persist, after wear and tear, only near the anterior and posterior margins. Growth stages prominent. Both valves with three cardinal teeth; in addition there is present in each valve a rough tooth-like area behind the beaks and immediately below the ligament; this area has the appearance of a supplementary posterior cardinal tooth which has been broken off. No laterals. Pallial sinus not deep, triangular. Margin grenulate. Colour a dirty white, light varnish-brown, dull grey or grey-brown. Inside of shell white, sometimes deep violet about the adductor muscle scars. -

DOGAMI Open-File Report O-86-06, the State of Scientific
"ABLE OF CONTENTS Page INTRODUCTION ..~**********..~...~*~~.~...~~~~1 GORDA RIDGE LEASE AREA ....................... 2 RELATED STUDIES IN THE NORTH PACIFIC .+,...,. 5 BYDROTHERMAL TEXTS ........................... 9 34T.4 GAPS ................................... r6 ACKNOWLEDGEMENT ............................. I8 APPENDIX 1. Species found on the Gorda Ridge or within the lease area . .. .. .. .. .. 36 RPPENDiX 2. Species found outside the lease area that may occur in the Gorda Ridge Lease area, including hydrothermal vent organisms .................................55 BENTHOS THE STATE OF SCIENTIFIC INFORMATION RELATING TO THE BIOLOGY AND ECOLOGY 3F THE GOUDA RIDGE STUDY AREA, NORTZEAST PACIFIC OCEAN: INTRODUCTION Presently, only two published studies discuss the ecology of benthic animals on the Gorda Sidge. Fowler and Kulm (19701, in a predominantly geolgg isal study, used the presence of sublittor31 and planktsnic foraminiferans as an indication of uplift of tfie deep-sea fioor. Their resuits showed tiac sedinenta ana foraminiferans are depositea in the Zscanaba Trough, in the southern part of the Corda Ridge, by turbidity currents with a continental origin. They list 22 species of fararniniferans from the Gorda Rise (See Appendix 13. A more recent study collected geophysical, geological, and biological data from the Gorda Ridge, with particular emphasis on the northern part of the Ridge (Clague et al. 19843. Geological data suggest the presence of widespread low-temperature hydrothermal activity along the axf s of the northern two-thirds of the Corda 3idge. However, the relative age of these vents, their present activity and presence of sulfide deposits are currently unknown. The biological data, again with an emphasis on foraminiferans, indicate relatively high species diversity and high density , perhaps assoc iated with widespread hydrotheraal activity. -

Benthic Data Sheet
DEMERSAL OTTER/BEAM TRAWL DATA SHEET RESEARCH VESSEL_____________________(1/20/13 Version*) CLASS__________________;DATE_____________;NAME:___________________________; DEVICE DETAILS_________ LOCATION (OVERBOARD): LAT_______________________; LONG______________________________ LOCATION (AT DEPTH): LAT_______________________; LONG_____________________________; DEPTH___________ LOCATION (START UP): LAT_______________________; LONG______________________________;.DEPTH__________ LOCATION (ONBOARD): LAT_______________________; LONG______________________________ TIME: IN______AT DEPTH_______START UP_______SURFACE_______.DURATION OF TRAWL________; SHIP SPEED__________; WEATHER__________________; SEA STATE__________________; AIR TEMP______________ SURFACE TEMP__________; PHYS. OCE. NOTES______________________; NOTES_______________________________ INVERTEBRATES Phylum Porifera Order Pennatulacea (sea pens) Class Calcarea __________________________________ Family Stachyptilidae Class Demospongiae (Vase sponge) _________________ Stachyptilum superbum_____________________ Class Hexactinellida (Hyalospongia- glass sponge) Suborder Subsessiliflorae Subclass Hexasterophora Family Pennatulidae Order Hexactinosida Ptilosarcus gurneyi________________________ Family Aphrocallistidae Family Virgulariidae Aphrocallistes vastus ______________________ Acanthoptilum sp. ________________________ Other__________________________________________ Stylatula elongata_________________________ Phylum Cnidaria (Coelenterata) Virgularia sp.____________________________ Other_______________________________________ -

Proceedings of the Academy of Natural Sciences of Philadelphia
32 PROCEEDINGS OF THE ACADEMY OF [1887. ON NEW GENEEIC FOKMS OF CRETACEOUS MOLLUSCA AND THEIE RELATION TO OTHER FORMS. BY CHARLES A. WHITE. Published by permission of the Director of the United States Geological Survey. The type species of the three generic forms which are described in this article ^ belong to the collections of Cretaceous fossils from Texas, which I am now preparing for publication in one of the memoirs of the U. S. Geological Survey. In their generic charac- teristics all three of them appear to be respectively identical with certain forms which have long been known, but which have been referred to other genera by different authors. The features which I now present as having generic value seem to have been overlooked by those authors, or, so far as they were observed, they were treated as specific characters. Two of these forms belong to the section Melininse of the family Aviculidse. The other is referred to the Crassatellidse, but it departs considerably from the typical section of that family. CRASSATELLIDJE. Genus STEARNSIA (gen. nov.). Shell compressed, subtrihedral or subcircular in marginal out- line; beaks small, closely approximate, prominent by reason of the abrupt sloping away of both the antero-and postero-dorsal borders; lunule and escutcheon both well defined and flattened or excavated; hinge strong, consisting of both cardinal and lateral teeth; cardinal teeth two in the left valve and three in the right; both posterior and anterior lateral teeth long and slender; posterior laterals two in the right valve and one in the left; anterior laterals two in the left valve and one in the right. -

Three Alien Molluscs from Iskenderun Bay (SE Turkey)
Aquatic Invasions (2006) Volume 1, Issue 2: 76-79 DOI 10.3391/ai.2006.1.2.4 © 2006 The Author(s) Journal compilation © 2006 REABIC (http://www.reabic.net) This is an Open Access article Research article Three alien molluscs from Iskenderun Bay (SE Turkey) Doğan Çeviker1 and Serhat Albayrak2* 1Itri Sokak No:2 34349 Balmumcu-Istanbul, Turkey E-mail: [email protected] 2Istanbul University, Faculty of Science, Department of Biology 34118 Vezneciler-Istanbul, Turkey E-mail: [email protected] *Corresponding author Received 26 April 2006; accepted in revised form 4 May 2006 Abstract This study reports the presence of three alien molluscs from Iskenderun Bay (SE Turkey). Amathina tricarinata (Linnaeus, 1767) and Petricola hemprichi Issel, 1869 have prior records from other regions of Mediterranean, but, Cardites akabana (Sturany, 1899) first recorded in this paper. Since all of them are present in the Red Sea or Suez Canal, they can be considered as Lessepsian immigrants. Key words: Mollusca, alien species, Mediterranean, Turkey Introduction that 88 % of the exotic molluscs are Lessepsian immigrants in the eastern Mediterranean (Galil The Mediterranean Sea hosts about 8500 species and Zenetos 2002). Detailed data about these species of macroscopic animals. This rich biodiversity, are available on the Internet (www.ciesm.org/atlas). representing 8-9 % of total species number of the Either Lessepsian or non-Lessepsian, many world’s seas, comprises temperate and sub- new non-indigenous species continue to enter the tropical elements together with endemic and Mediterranean. alien species (Zenetos et al. 2002). The eastern Mediterranean is most vulnerable The introduction of alien species (also known to invasion and should be continuously as exotic, introduced or non-native species) into monitored. -

Eoursivivas Cultriformis
Page 118 The Veliger, Vol. 47, No. 2 Figures 40-53. Specimens coated with ammonium chloride. Figures 40-45. Panzacorbula Squires & Saul, gen. nov. pozo (Dailey & Popenoe, 1966). Figure 40. Holotype LACMIP 8916, LACMIP loc. 23774, left valve, X2.2. Figure 41. Paratype LACMIP 8918, LACMIP loc. 23774, left-valve interior, X2.1. Figure 42. Paratype LACMIP 8917, LACMIP loc. 23774, right valve, X2.1. Figure 43. Hypotype LACMIP 13124, LACMIP loc. 10667, immature right valve, X4.1. Figure 44. Paratype LACMIP 8917, LACMIP loc. 23774, right-valve interior, X2. Figure 45. Holotype LACMIP 8916, LACMIP loc. 23774, dorsal view, X2. Figures 46-49. Eoursivivas cultri- formis (Gabb, 1864). Figure 46. Hypotype LACMIP 13125, LACMIP loc. 26345, left valve, X2.6. Figure 47. Hypotype LACMIP 13126, LACMIP loc. 26345, left valve, X5.1. Figure 48. Lectotype UCMP 11945a, CGS loc. 144, right valve, X5.2. Figure 49. Hypotype LACMIP 13127, LACMIP loc. 26345, right valve, X2.3. Figures 50-53. Caestocorbula cavus Squires & Saul, sp. nov., UCMP loc. B- 5611. Figure 50. Paratype UCMP 155540, left valve, XI 3.7. Figures 51-53. Holotype UCMP 155539, X7. Figure 51. Left valve. Figure 52. Right valve. Figure 53. Dorsal view. R. L. Squires & L. R. Saul, 2004 Page 119 Diagnosis: Same as for genus. Discussion: This study of Dailey and Popenoe's species Description: Shell medium (maximum length 21.7 mm); is based on 124 specimens (including the type material): moderately thick. Valves subpyriform to trigonal elon- 96 right valves, 25 left valves, and three pairs of con- gate, inflated (right valve more inflated than left valve), joined valves. -

Guide to Estuarine and Inshore Bivalves of Virginia
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1968 Guide to Estuarine and Inshore Bivalves of Virginia Donna DeMoranville Turgeon College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Marine Biology Commons, and the Oceanography Commons Recommended Citation Turgeon, Donna DeMoranville, "Guide to Estuarine and Inshore Bivalves of Virginia" (1968). Dissertations, Theses, and Masters Projects. Paper 1539617402. https://dx.doi.org/doi:10.25773/v5-yph4-y570 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. GUIDE TO ESTUARINE AND INSHORE BIVALVES OF VIRGINIA A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Arts LIBRARY o f the VIRGINIA INSTITUTE Of MARINE. SCIENCE. By Donna DeMoranville Turgeon 1968 APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts jfitw-f. /JJ'/ 4/7/A.J Donna DeMoranville Turgeon Approved, August 1968 Marvin L. Wass, Ph.D. P °tj - D . dvnd.AJlLJ*^' Jay D. Andrews, Ph.D. 'VL d. John L. Wood, Ph.D. William J. Hargi Kenneth L. Webb, Ph.D. ACKNOWLEDGEMENTS The author wishes to express sincere gratitude to her major professor, Dr. -

Analysis of Phylogeny and Specificity by 16S Rrna Sequences D
JOURNAL OF BACTERIOLOGY, June 1988, p. 2506-2510 Vol. 170, No. 6 0021-9193/88/062506-05$02.00/0 Copyright C 1988, American Society for Microbiology Sulfur-Oxidizing Bacterial Endosymbionts: Analysis of Phylogeny and Specificity by 16S rRNA Sequences D. L. DISTEL,1* D. J. LANE,2t G. J. OLSEN,2 S. J. GIOVANNONI,2 B. PACE,2 N. R. PACE,2 D. A. STAHL,3 AND H. FELBECK' Scripps Institution of Oceanography, University of California, San Diego, La Jolla, California 920931; Department of Biology, Indiana University, Bloomington, Indiana 474052; and Department of Veterinary Pathobiology, University ofIllinois, Urbana, Illinois 618083 Received 9 December 1987/Accepted 9 March 1988 The 16S rRNAs from the bacterial endosymbionts of six marine invertebrates from diverse environments were isolated and partially sequenced. These symbionts included the trophosome symbiont ofRiftia pachyptila, the gill symbionts of Calyptogena magnffica and Bathymodiolus thermophilus (from deep-sea hydrothermal vents), and the gill symbionts of Lucinoma annulata, Lucinoma aequizonata, and Codakia orbicularis (from relatively shallow coastal environments). Only one type of bacterial 16S rRNA was detected in each symbiosis. Using nucleotide sequence comparisons, we showed that each of the bacterial symbionts is distinct from the others and that all fall within a limited domain of the gamma subdivision of the purple bacteria (one of the major eubacterial divisions previously defined by 16S rRNA analysis [C. R. Woese, Microbiol. Rev. 51:221-271, 1987]). Two host specimens were analyzed in five of the symbioses; in each case, identical bacterial rRNA sequences were obtained from conspecific host specimens. These data indicate that the symbioses examined are species specific and that the symbiont species are unique to and invariant within their respective host species. -

Articles and Detrital Matter
Biogeosciences, 7, 2851–2899, 2010 www.biogeosciences.net/7/2851/2010/ Biogeosciences doi:10.5194/bg-7-2851-2010 © Author(s) 2010. CC Attribution 3.0 License. Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem E. Ramirez-Llodra1, A. Brandt2, R. Danovaro3, B. De Mol4, E. Escobar5, C. R. German6, L. A. Levin7, P. Martinez Arbizu8, L. Menot9, P. Buhl-Mortensen10, B. E. Narayanaswamy11, C. R. Smith12, D. P. Tittensor13, P. A. Tyler14, A. Vanreusel15, and M. Vecchione16 1Institut de Ciencies` del Mar, CSIC. Passeig Mar´ıtim de la Barceloneta 37-49, 08003 Barcelona, Spain 2Biocentrum Grindel and Zoological Museum, Martin-Luther-King-Platz 3, 20146 Hamburg, Germany 3Department of Marine Sciences, Polytechnic University of Marche, Via Brecce Bianche, 60131 Ancona, Italy 4GRC Geociencies` Marines, Parc Cient´ıfic de Barcelona, Universitat de Barcelona, Adolf Florensa 8, 08028 Barcelona, Spain 5Universidad Nacional Autonoma´ de Mexico,´ Instituto de Ciencias del Mar y Limnolog´ıa, A.P. 70-305 Ciudad Universitaria, 04510 Mexico,` Mexico´ 6Woods Hole Oceanographic Institution, MS #24, Woods Hole, MA 02543, USA 7Integrative Oceanography Division, Scripps Institution of Oceanography, La Jolla, CA 92093-0218, USA 8Deutsches Zentrum fur¨ Marine Biodiversitatsforschung,¨ Sudstrand¨ 44, 26382 Wilhelmshaven, Germany 9Ifremer Brest, DEEP/LEP, BP 70, 29280 Plouzane, France 10Institute of Marine Research, P.O. Box 1870, Nordnes, 5817 Bergen, Norway 11Scottish Association for Marine Science, Scottish Marine Institute, Oban, -

Chapter I Taxonomy
THE AMERICAN OYSTER CRASSOSTREA VIRGINICA GMELIN By PAUL S. GALTSOFF, Fishery Biologist BUREAU OF COMMERCIAL FISHERIES CHAPTER I TAXONOMY Page This broad characterization included a number Taxonomic characters _ 4 SheIL _ 4 of genera such as scallops, pen shells (Pinnidae), Anatomy _ 4 Sex and spawnlng _ limas (Limidae) and other mollusks which ob 4 Habitat _ 5 viously are not oysters. In the 10th edition of Larvll! shell (Prodlssoconch) _ 6 "Systema Naturae," Linnaeus (1758) wrote: The genera of living oysters _ 6 Genus 08trea _ 6 "Ostreae non orones, imprimis Pectines, ad Genus Cra8808trea _ 7 Genus Pycnodonte _ cardinem interne fulcis transversis numerosis 7 Bibliography _ 14 parallelis in utraque testa oppositis gaudentiquae probe distinguendae ab Areis polypleptoginglymis, The family Ostreidae consists of a large number cujus dentes numerosi alternatim intrant alterius of edibleand nonedible oysters. Their distribution sinus." Le., not all are oysters, in particular the is confined to a broad belt of coastal waters within scallops, which have many parallel ribs running the latitudes 64° N. and 44° S. With few excep crosswise inward toward the hinge on each shell tions oysters thrive in shallow water, their vertical on opposite sides; these should properly be dis distribution extending from a level approximately tinguished from Area polyleptoginglymis whose halfway between high and low tide levels to a many teeth alternately enter between the teeth depth of about 100 feet. Commercially exploited of the other side. oyster beds are rarely found below a depth of 40 In the same publication the European flat feet. oyster, Ostrea edulis, is described as follows: The· name "Ostrea" was given by Linnaeus "Vulgo Ostrea dictae edulis.