<I>Fimbria Fimbriata</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Field Identification Guide to the Living Marine Resources In
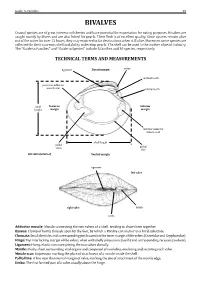
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

FAO Fisheries & Aquaculture
Food and Agriculture Organization of the United Nations Fisheries and for a world without hunger Aquaculture Department Cultured Aquatic Species Information Programme Mercenaria mercenaria (Linnaeus, 1758) I. Identity V. Status And Trends a. Biological Features VI. Main Issues b. Images Gallery a. Responsible Aquaculture Practices II. Profile VII. References a. Historical Background a. Related Links b. Main Producer Countries c. Habitat And Biology III. Production a. Production Cycle b. Production Systems c. Diseases And Control Measures IV. Statistics a. Production Statistics b. Market And Trade Identity Mercenaria mercenaria Linnaeus, 1758 [Veneridae] FAO Names: En - Northern quahog(=Hard clam), Fr - Praire, Es - Chirla mercenaria Biological features Shell solid, equivalve; inequilateral, beaks in the front half of the shell; broadly oval in outline. Ligament a deeply inset, dark brown elliptical band, behind the beaks reaching half-way to the posterior margin. Lunule well defined, broad, heart-shaped. Escutcheon indistinct. Sculpture of concentric lines, raised here and there into ridges, and fine radiating lines. In young specimens the ridges are present all over the shell but in the adult they persist, after wear and tear, only near the anterior and posterior margins. Growth stages prominent. Both valves with three cardinal teeth; in addition there is present in each valve a rough tooth-like area behind the beaks and immediately below the ligament; this area has the appearance of a supplementary posterior cardinal tooth which has been broken off. No laterals. Pallial sinus not deep, triangular. Margin grenulate. Colour a dirty white, light varnish-brown, dull grey or grey-brown. Inside of shell white, sometimes deep violet about the adductor muscle scars. -

Food Handling and Mastication in the Carp (Cyprinus Carpio L.)
KJAJA2Ö*)O)Ó Food handling and mastication in the carp (Cyprinus carpio L.) ««UB***1* WIT1 TUOSW«- «* omslag tekening : Wim Valen -2- Promotor: dr. J.W.M. Osse, hoogleraar in de algemene dierkunde ^iJOttO^ 1oID Ferdinand A. Sibbing FOOD HANDLING AND MASTICATION IN THE CARP (Cyprinus carpio L.) Proefschrift ter verkrijging van de graad van doctor in de landbouwwetenschappen, op gezag van de rector magnificus, dr. C.C.Oosterlee, in het openbaar te verdedigen op dinsdag 11 december 1984 des namiddags te vier uur in de aula van de Landbouwhogeschool te Wageningen. l^V-, ^v^biS.oa BIBLIOTHEEK ^LANDBOUWHOGESCHOOL WAGENINGEN ^/y/of^OÏ, fOtO STELLINGEN 1. De taakverdeling tussen de kauwspieren van de karper is analoog aan die tussen vliegspieren van insekten: grote lichaamsspieren leveren indirekt het vermogen, terwijl direkt aangehechte kleinere spieren de beweging vooral sturen. Deze analogie komt voort uit architekturale en kinematische principes. 2. Naamgeving van spieren op grond van hun verwachte rol (b.v. levator, retractor) zonder dat deze feitelijk is onderzocht leidt tot lang doorwerkende misvattingen over hun funktie en geeft blijk van een onderschatting van de plasticiteit waarmee spieren worden ingezet. Een nomenclatuur die gebaseerd is op origo en insertie van de spier verdient de voorkeur. 3. De uitstulpbaarheid van de gesloten bek bij veel cypriniden maakt een getrapte zuivering van het voedsel mogelijk en speelt zo een wezenlijke rol in de selektie van bodemvoedsel. Dit proefschrift. 4. Op grond van de vele funkties die aan slijm in biologische systemen worden toegeschreven is meer onderzoek naar zijn chemische en fysische eigenschappen dringend gewenst. Dit proefschrift. -

Cloaked Bivalve Oocytes
The Scientific Naturalist Ecology, 100(12), 2019, e02818 © 2019 by the Ecological Society of America entirely benthic development (Ockelman 1958, Collin and Giribet 2010). The next question is, obviously: What makes it? Because the bivalve germinal epithelium has no secre- tory cells, and no auxiliary cells have been observed con- Cloaked bivalve oocytes: lessons in structing such a feature around developing oocytes, the evolution, ecology, and scientific most likely origin is the oocyte itself—and this has also been reported in unpublished work (cited in Gros et al. awareness 1997). Histological sections show a thin cloak around young oocytes (not shown), and a very thick one around 1 PETER G. BENINGER AND DAPHNE CHEREL mature oocytes (Fig. 1B). Manuscript received 20 March 2019; revised 15 May 2019; With respect to evolutionary lessons, coated oocytes accepted 17 June 2019. Corresponding Editor: John Pastor. have been observed in species from four of the six Faculte des Sciences, Universite de Nantes, 2 rue de la subclasses of the Bivalvia. There appears to be no Houssiniere, 44322 Nantes Cedex, France. taxonomic or evolutionary pattern in their occurrence; 1 E-mail: [email protected] they are found both in the primitive Cryptodonta and in the much later Heterodonta and Anomalodesmata Citation: Beninger, P. G., and D. Cherel. 2019. Cloaked (Table 1). In each of these subclasses, there are also bivalve oocytes: lessons in evolution, ecology, and scien- many species without coated oocytes, even within the tific awareness. Ecology 100(12):e02818. 10.1002/ecy.2818 same family (e.g., Pectinidae and Veneridae). The possi- bility that this is the result of multiple convergent evolu- Key words: bivalves; coat; mucopolysaccharides; oocytes; scien- tions of an identical character seems so remote as to be tific awareness. -

Copyrighted Material
319 Index a oral cavity 195 guanocytes 228, 231, 233 accessory sex glands 125, 316 parasites 210–11 heart 235 acidophils 209, 254 pharynx 195, 197 hemocytes 236 acinar glands 304 podocytes 203–4 hemolymph 234–5, 236 acontia 68 pseudohearts 206, 208 immune system 236 air sacs 305 reproductive system 186, 214–17 life expectancy 222 alimentary canal see digestive setae 191–2 Malpighian tubules 232, 233 system taxonomy 185 musculoskeletal system amoebocytes testis 214 226–9 Cnidaria 70, 77 typhlosole 203 nephrocytes 233 Porifera 28 antennae nervous system 237–8 ampullae 10 Decapoda 278 ocelli 240 Annelida 185–218 Insecta 301, 315 oral cavity 230 blood vessels 206–8 Myriapoda 264, 275 ovary 238 body wall 189–94 aphodus 38 pedipalps 222–3 calciferous glands 197–200 apodemes 285 pharynx 230 ciliated funnel 204–5 apophallation 87–8 reproductive system 238–40 circulatory system 205–8 apopylar cell 26 respiratory system 236–7 clitellum 192–4 apopyle 38 silk glands 226, 242–3 coelomocytes 208–10 aquiferous system 21–2, 33–8 stercoral sac 231 crop 200–1 Arachnida 221–43 sucking stomach 230 cuticle 189 biomedical applications 222 taxonomy 221 diet 186–7 body wall 226–9 testis 239–40 digestive system 194–203 book lungs 236–7 tracheal tube system 237 dissection 187–9 brain 237 traded species 222 epidermis 189–91 chelicera 222, 229 venom gland 241–2 esophagus 197–200 circulatory system 234–6 walking legs 223 excretory system 203–5 COPYRIGHTEDconnective tissue 228–9 MATERIALzoonosis 222 ganglia 211–13 coxal glands 232, 233–4 archaeocytes 28–9 giant nerve -

Embryonic and Larval Development of Ensis Arcuatus (Jeffreys, 1865) (Bivalvia: Pharidae)
EMBRYONIC AND LARVAL DEVELOPMENT OF ENSIS ARCUATUS (JEFFREYS, 1865) (BIVALVIA: PHARIDAE) FIZ DA COSTA, SUSANA DARRIBA AND DOROTEA MARTI´NEZ-PATIN˜O Centro de Investigacio´ns Marin˜as, Consellerı´a de Pesca e Asuntos Marı´timos, Xunta de Galicia, Apdo. 94, 27700 Ribadeo, Lugo, Spain (Received 5 December 2006; accepted 19 November 2007) ABSTRACT The razor clam Ensis arcuatus (Jeffreys, 1865) is distributed from Norway to Spain and along the British coast, where it lives buried in sand in low intertidal and subtidal areas. This work is the first study to research the embryology and larval development of this species of razor clam, using light and scanning electron microscopy. A new method, consisting of changing water levels using tide simulations with brief Downloaded from https://academic.oup.com/mollus/article/74/2/103/1161011 by guest on 23 September 2021 dry periods, was developed to induce spawning in this species. The blastula was the first motile stage and in the gastrula stage the vitelline coat was lost. The shell field appeared in the late gastrula. The trocho- phore developed by about 19 h post-fertilization (hpf) (198C). At 30 hpf the D-shaped larva showed a developed digestive system consisting of a mouth, a foregut, a digestive gland followed by an intestine and an anus. Larvae spontaneously settled after 20 days at a length of 378 mm. INTRODUCTION following families: Mytilidae (Redfearn, Chanley & Chanley, 1986; Fuller & Lutz, 1989; Bellolio, Toledo & Dupre´, 1996; Ensis arcuatus (Jeffreys, 1865) is the most abundant species of Hanyu et al., 2001), Ostreidae (Le Pennec & Coatanea, 1985; Pharidae in Spain. -

Are the Traditional Medical Uses of Muricidae Molluscs Substantiated by Their Pharmacological Properties and Bioactive Compounds?
Mar. Drugs 2015, 13, 5237-5275; doi:10.3390/md13085237 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Review Are the Traditional Medical Uses of Muricidae Molluscs Substantiated by Their Pharmacological Properties and Bioactive Compounds? Kirsten Benkendorff 1,*, David Rudd 2, Bijayalakshmi Devi Nongmaithem 1, Lei Liu 3, Fiona Young 4,5, Vicki Edwards 4,5, Cathy Avila 6 and Catherine A. Abbott 2,5 1 Marine Ecology Research Centre, School of Environment, Science and Engineering, Southern Cross University, G.P.O. Box 157, Lismore, NSW 2480, Australia; E-Mail: [email protected] 2 School of Biological Sciences, Flinders University, G.P.O. Box 2100, Adelaide 5001, Australia; E-Mails: [email protected] (D.R.); [email protected] (C.A.A.) 3 Southern Cross Plant Science, Southern Cross University, G.P.O. Box 157, Lismore, NSW 2480, Australia; E-Mail: [email protected] 4 Medical Biotechnology, Flinders University, G.P.O. Box 2100, Adelaide 5001, Australia; E-Mails: [email protected] (F.Y.); [email protected] (V.E.) 5 Flinders Centre for Innovation in Cancer, Flinders University, G.P.O. Box 2100, Adelaide 5001, Australia 6 School of Health Science, Southern Cross University, G.P.O. Box 157, Lismore, NSW 2480, Australia; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +61-2-8201-3577. Academic Editor: Peer B. Jacobson Received: 2 July 2015 / Accepted: 7 August 2015 / Published: 18 August 2015 Abstract: Marine molluscs from the family Muricidae hold great potential for development as a source of therapeutically useful compounds. -

Download Complete Work
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS Laseron, C. F., 1956. A revision of the New South Wales Leptonidae (Mollusca: Pelecypoda). Records of the Australian Museum 24(2): 7–22. [23 November 1956]. doi:10.3853/j.0067-1975.24.1956.640 ISSN 0067-1975 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia 7 A REVISION OF THE NEW SOUTH WALES LEPTONIDAE (MOLLUSCA: Pelecypoda) (l!"igs. 1-27) By CHARLES F. LASERON, F.R.Z.S. (This research has been assisted by a gTant from tbe Science and Industry Endowment Fund.) INTRODUCTION. Th~ group of bivalves dealt with in this paper has been classified differently by AustralasIan cO~lChologists. Hedley in his check list, 1918, used Leptonidae as a family name.. Powell In "~~ellfish of New Zealand", 1937, divided the group into two families Lasa81dae and Erycll1ldae. Ootton and Godfrey, "The Mollusca of South Australia", 1938, used Leptonacea as a superfamily, divided into two families, Leptonidae and Montacutidae. POivell again, in a second edition, 1946, reverted to the single family Leptonidae . .Th.at arrangement is followed here. The group, whether considered as a family or superfamlly, seems a natural one, and the characters, both anatomical and of the shell, are reasonably definable. Many of the genera are nestling, others are reputecl to be either commensal or parasitic, but the latter habits have Hot been 1l0ticeil in any of the Peronian forms. -

Proceedings of the Academy of Natural Sciences of Philadelphia
32 PROCEEDINGS OF THE ACADEMY OF [1887. ON NEW GENEEIC FOKMS OF CRETACEOUS MOLLUSCA AND THEIE RELATION TO OTHER FORMS. BY CHARLES A. WHITE. Published by permission of the Director of the United States Geological Survey. The type species of the three generic forms which are described in this article ^ belong to the collections of Cretaceous fossils from Texas, which I am now preparing for publication in one of the memoirs of the U. S. Geological Survey. In their generic charac- teristics all three of them appear to be respectively identical with certain forms which have long been known, but which have been referred to other genera by different authors. The features which I now present as having generic value seem to have been overlooked by those authors, or, so far as they were observed, they were treated as specific characters. Two of these forms belong to the section Melininse of the family Aviculidse. The other is referred to the Crassatellidse, but it departs considerably from the typical section of that family. CRASSATELLIDJE. Genus STEARNSIA (gen. nov.). Shell compressed, subtrihedral or subcircular in marginal out- line; beaks small, closely approximate, prominent by reason of the abrupt sloping away of both the antero-and postero-dorsal borders; lunule and escutcheon both well defined and flattened or excavated; hinge strong, consisting of both cardinal and lateral teeth; cardinal teeth two in the left valve and three in the right; both posterior and anterior lateral teeth long and slender; posterior laterals two in the right valve and one in the left; anterior laterals two in the left valve and one in the right. -

Reproductive Characteristics and Strategies of Reducing-System Bivalves
Comparative Biochemistry and Physiology Part A 126 (2000) 1–16 www.elsevier.com/locate/cbpa Review Reproductive characteristics and strategies of reducing-system bivalves Marcel Le Pennec a, Peter G. Beninger b,* a Institut Uni6ersitaire Europe´endelaMer, Place Nicolas Copernic, Technopoˆle Brest-Iroise, 29280 Plouzane´, France b Laboratoire de Biologie Marine, Faculte´ des Sciences, Uni6ersite´ de Nantes, 44322 Nantes ce´dex, France Received 23 September 1999; received in revised form 15 February 2000; accepted 25 February 2000 Abstract The reproductive biology of Type 3 reducing-system bivalves (those whose pallial cavity is irrigated with water rich in reducing substances) is reviewed, with respect to size-at-maturity, sexuality, reproductive cycle, gamete size, symbiont transmission, and larval development/dispersal strategies. The pattern which emerges from the fragmentary data is that these organisms present reproductive particularities associated with their habitat, and with their degree of reliance on bacterial endosymbionts. A partial exception to this pattern is the genus Bathymodiolus, which also presents fewer trophic adaptations to the reducing environment, suggesting a bivalent adaptive strategy. A more complete understand- ing of the reproductive biology of Type 3 bivalves requires much more data, which may not be feasible for some aspects in the deep-sea species. © 2000 Elsevier Science Inc. All rights reserved. Keywords: Reproduction; Bivalves; Reducing; Hydrothermal 1. Introduction 1979; Jannasch 1985; Smith 1985; Morton 1986; Reid and Brand, 1986; Distel and Felbeck 1987; Interest in the biology of marine reducing sys- Diouris et al. 1989; Tunnicliffe 1991; Le Pennec et tems has surged since the discovery of deep-sea al., 1995a). In contrast, general principles of the vents and associated fauna (Corliss et al., 1979). -

SPIXIANA ©Zoologische Staatssammlung München
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Spixiana, Zeitschrift für Zoologie Jahr/Year: 1986 Band/Volume: 009 Autor(en)/Author(s): Barash Al., Danin Z. Artikel/Article: Further additions to the knowledge of Indo-Pacific Mollusca in the Mediterranean Sea (Lessepsian migrants) 117-141 ©Zoologische Staatssammlung München;download: http://www.biodiversitylibrary.org/; www.biologiezentrum.at SPIXIANA ©Zoologische Staatssammlung München;download: http://www.biodiversitylibrary.org/; www.biologiezentrum.at Today we are able to present an additional report on 29 Indo-Pacific species in the Mediterranean which have not been discussed in the general reports of 1948-1977 mentioned above. Eight species are recorded for the first time; 3 species were mentioned by name only (but not discussed) in the article by Barash & Danin (1982: 107) and 18 species have been dealt with in articles on individual Indo-Pacific immigrants. Along with the 29 species not yet discussed in earher reports, 4 species, which have been treated pre- viously are included in this paper for supplementary data (see general remarks, p. 130ff.). This report is based on material obtained from various sources, as follows: A great deal of dredging was carried out during 1974-1977 by the late Prof. Ch. Lewinsohn (Tel Aviv University) and his assistants M. Tom and B. Galil. They worked in the infralittoral zone in north and south Israel and opposite the Mediterranean coast of north Sinai. Among the material coUected by them the nudibranch Plocamopherus ocellatus is noteworthy because of its power of luminescence (O'Do- NOGHUE 1929: 808). -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North