Identification of Genes Related to Beak Deformity of Chickens Using Digital Gene Expression Profiling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
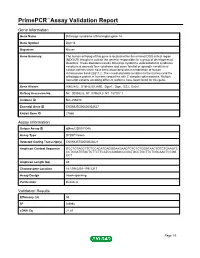
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name DiGeorge syndrome critical region gene 14 Gene Symbol Dgcr14 Organism Mouse Gene Summary The human ortholog of this gene is located within the minimal DGS critical region (MDGCR) thought to contain the gene(s) responsible for a group of developmental disorders. These disorders include DiGeorge syndrome velocardiofacial syndrome conotruncal anomaly face syndrome and some familial or sporadic conotruncal cardiac defects which have been associated with microdeletion of human chromosome band 22q11.2. The encoded protein localizes to the nucleus and the orthologous protein in humans co-purifies with C complex spliceosomes. Multiple transcript variants encoding different isoforms have been found for this gene. Gene Aliases AI462402, D16H22S1269E, Dgcr1, Dgsi, ES2, Es2el RefSeq Accession No. NC_000082.6, NT_039624.8, NT_187007.1 UniGene ID Mm.256480 Ensembl Gene ID ENSMUSG00000003527 Entrez Gene ID 27886 Assay Information Unique Assay ID qMmuCID0011048 Assay Type SYBR® Green Detected Coding Transcript(s) ENSMUST00000003621 Amplicon Context Sequence GCCTGTAGCTTCTCCACATCAGGGAAGAAGTCTCTCTGGATAACTGTCTGAAGTC CCTCGATGTACTCTTCTTCATCCAGGACCCGCTGCCTGCTTCTCGCAACTCCGG CCT Amplicon Length (bp) 82 Chromosome Location 16:17910201-17911217 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 95 R2 0.9994 cDNA Cq 21.87 Page 1/5 PrimePCR™Assay Validation Report cDNA Tm (Celsius) 79.5 gDNA Cq 23.94 Specificity (%) 100 Information to assist with data interpretation is provided -

CRNKL1 Is a Highly Selective Regulator of Intron-Retaining HIV-1 and Cellular Mrnas
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.04.934927; this version posted February 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 CRNKL1 is a highly selective regulator of intron-retaining HIV-1 and cellular mRNAs 2 3 4 Han Xiao1, Emanuel Wyler2#, Miha Milek2#, Bastian Grewe3, Philipp Kirchner4, Arif Ekici4, Ana Beatriz 5 Oliveira Villela Silva1, Doris Jungnickl1, Markus Landthaler2,5, Armin Ensser1, and Klaus Überla1* 6 7 1 Institute of Clinical and Molecular Virology, University Hospital Erlangen, Friedrich-Alexander 8 Universität Erlangen-Nürnberg, Erlangen, Germany 9 2 Berlin Institute for Medical Systems Biology, Max-Delbrück-Center for Molecular Medicine in the 10 Helmholtz Association, Robert-Rössle-Strasse 10, 13125, Berlin, Germany 11 3 Department of Molecular and Medical Virology, Ruhr-University, Bochum, Germany 12 4 Institute of Human Genetics, University Hospital Erlangen, Friedrich-Alexander Universität Erlangen- 13 Nürnberg, Erlangen, Germany 14 5 IRI Life Sciences, Institute für Biologie, Humboldt Universität zu Berlin, Philippstraße 13, 10115, Berlin, 15 Germany 16 # these two authors contributed equally 17 18 19 *Corresponding author: 20 Prof. Dr. Klaus Überla 21 Institute of Clinical and Molecular Virology, University Hospital Erlangen 22 Friedrich-Alexander Universität Erlangen-Nürnberg 23 Schlossgarten 4, 91054 Erlangen 24 Germany 25 Tel: (+49) 9131-8523563 26 e-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.04.934927; this version posted February 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

Análise Integrativa De Perfis Transcricionais De Pacientes Com
UNIVERSIDADE DE SÃO PAULO FACULDADE DE MEDICINA DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM GENÉTICA ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Ribeirão Preto – 2012 ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Tese apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo para obtenção do título de Doutor em Ciências. Área de Concentração: Genética Orientador: Prof. Dr. Eduardo Antonio Donadi Co-orientador: Prof. Dr. Geraldo A. S. Passos Ribeirão Preto – 2012 AUTORIZO A REPRODUÇÃO E DIVULGAÇÃO TOTAL OU PARCIAL DESTE TRABALHO, POR QUALQUER MEIO CONVENCIONAL OU ELETRÔNICO, PARA FINS DE ESTUDO E PESQUISA, DESDE QUE CITADA A FONTE. FICHA CATALOGRÁFICA Evangelista, Adriane Feijó Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. Ribeirão Preto, 2012 192p. Tese de Doutorado apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo. Área de Concentração: Genética. Orientador: Donadi, Eduardo Antonio Co-orientador: Passos, Geraldo A. 1. Expressão gênica – microarrays 2. Análise bioinformática por module maps 3. Diabetes mellitus tipo 1 4. Diabetes mellitus tipo 2 5. Diabetes mellitus gestacional FOLHA DE APROVAÇÃO ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. -

Análise Correlacional Entre a Expressão Dos Fatores De Splicing E a Ocorrência De Splicing Alternativo Em Tecidos Humanos E De Camundongos
ANÁLISE CORRELACIONAL ENTRE A EXPRESSÃO DOS FATORES DE SPLICING E A OCORRÊNCIA DE SPLICING ALTERNATIVO EM TECIDOS HUMANOS E DE CAMUNDONGOS JULIO CÉSAR NUNES Dissertação apresentada à Fundação Antônio Prudente para a obtenção do título de Mestre em Ciências Área de Concentração: Oncologia Orientador: Dr. Sandro José de Souza São Paulo 2008 Livros Grátis http://www.livrosgratis.com.br Milhares de livros grátis para download. FICHA CATALOGRÁFICA Preparada pela Biblioteca da Fundação Antônio Prudente Nunes, Julio César Análise correlacional entre a expressão dos fatores de splicing e a ocorrência de splicing alternativo em tecidos humanos e de camundongos / Julio César Nunes – São Paulo, 2008. 79p. Dissertação (Mestrado) - Fundação Antônio Prudente. Curso de Pós-Graduação em Ciências - Área de concentração: Oncologia. Orientador: Sandro José Souza Descritores: 1. SPLICING ALTERNATIVO 2. BIOLOGIA MOLECULAR COMPUTACIONAL 3. CÂNCER 4. GENOMICA. AGRADECIMENTOS Agradeço à FAPESP e CAPES pela bolsa de Mestrado. Ao Sandro José de Souza agradeço toda orientação e conhecimento oferecido. Meus especiais agradecimentos ao Pedro Alexandre Favoretto Galante que dedicou atenção a minha formação no processo de Pós-Graduação na Fundação Antônio Prudente, bem como pela sua oficiosa co-orientação ao projeto de pesquisa. À grande família e amigos pela dedicação e incentivo a minha formação acadêmica. À Fundação Antônio Prudente, Hospital do Câncer e Instituto Ludwig de Pesquisa sobre o Câncer dedico os meus nobres agradecimentos finais. RESUMO Nunes JC. Análise correlacional entre a expressão dos fatores de splicing e a ocorrência de splicing alternativo em tecidos humanos e de camundongos. São Paulo; 2007. [Dissertacão de Mestrado - Fundação Antônio Prudente] Splicing alternativo desempenha uma significante função no aumento da complexidade genômica, produzindo um extenso número de mRNA e isoformas protéicas. -

DGCR6 at the Proximal Part of the Digeorge Critical Region Is Involved in Conotruncal Heart Defects
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Institutional Repository : the EHIME area OPEN Citation: Human Genome Variation (2015) 2, 15004; doi:10.1038/hgv.2015.4 © 2015 The Japan Society of Human Genetics All rights reserved 2054-345X/15 www.nature.com/hgv ARTICLE DGCR6 at the proximal part of the DiGeorge critical region is involved in conotruncal heart defects Wenming Gao1, Takashi Higaki1, Minenori Eguchi-Ishimae1, Hidehiko Iwabuki1, Zhouying Wu1, Eiichi Yamamoto2, Hidemi Takata1, Masaaki Ohta1, Issei Imoto3, Eiichi Ishii1 and Mariko Eguchi1 Cardiac anomaly is one of the hallmarks of DiGeorge syndrome (DGS), observed in approximately 80% of patients. It often shows a characteristic morphology, termed as conotruncal heart defects. In many cases showing only the conotruncal heart defect, deletion of 22q11.2 region cannot be detected by fluorescence in situ hybridization (FISH), which is used to detect deletion in DGS. We investigated the presence of genomic aberrations in six patients with congenital conotruncal heart defects, who show no deletion at 22q11.2 in an initial screening by FISH. In these patients, no abnormalities were identified in the coding region of the TBX1 gene, one of the key genes responsible for the phenotype of DGS. However, when copy number alteration was analyzed by high-resolution array analysis, a small deletion or duplication in the proximal end of DiGeorge critical region was detected in two patients. The affected region contains the DGCR6 and PRODH genes. DGCR6 has been reported to affect the expression of the TBX1 gene. Our results suggest that altered dosage of gene(s) other than TBX1, possibly DGCR6, may also be responsible for the development of conotruncal heart defects observed in patients with DGS and, in particular, in those with stand-alone conotruncal heart defects. -

A Collection of Pre-Mrna Splicing Mutants in Arabidopsis Thaliana
G3: Genes|Genomes|Genetics Early Online, published on April 7, 2020 as doi:10.1534/g3.119.400998 1 1 A collection of pre-mRNA splicing mutants in Arabidopsis thaliana 2 3 4 Tatsuo Kanno1,a, Peter Venhuizen2,a, Ming-Tsung Wu3, Phebe Chiou1, Chia-Liang Chang1, 5 Maria Kalyna2,c, Antonius J.M. Matzke1,c and Marjori Matzke1,c 6 7 1Institute of Plant and Microbial Biology, Academia Sinica, 128, Sec. 2, Academia Rd., Nangang 8 District, Taipei, 11529 Taiwan 9 10 2Department of Applied Genetics and Cell Biology, University of Natural Resources and Life 11 Sciences - BOKU, Muthgasse 18, 1190 Vienna, Austria 12 13 3Department of Plant Sciences, University of Cambridge, Downing Street, CB2 3EA Cambridge, 14 UK 15 16 athese authors should be regarded as joint first authors 17 18 19 cCorresponding authors: 20 Antonius J.M. Matzke ([email protected]) 21 Tel: +886-2787-1135 22 Marjori Matzke ([email protected]) 23 Tel: +886-2787-1135 24 Maria Kalyna ([email protected]) 25 Tel: +43-1-47654-94112 26 27 28 29 30 31 32 © The Author(s) 2020. Published by the Genetics Society of America. 2 33 34 Running title: Arabidopsis pre-mRNA splicing mutants 35 36 Key words: Arabidopsis thaliana, CBP80. miRNAs, mutant screen, pre-mRNA splicing 37 38 39 40 Corresponding authors: 41 Antonius J.M. Matzke ([email protected]), Institute of Plant and Microbial 42 Biology, Academia Sinica, 128, Sec. 2, Academia Rd., Nangang District, Taipei, 11529 Taiwan 43 Tel: +886-2787-1135 44 45 Marjori Matzke ([email protected]), Institute of Plant and Microbial Biology, 46 Academia Sinica, 128, Sec. -

A Catalog of Hemizygous Variation in 127 22Q11 Deletion Patients
A catalog of hemizygous variation in 127 22q11 deletion patients. Matthew S Hestand, KU Leuven, Belgium Beata A Nowakowska, KU Leuven, Belgium Elfi Vergaelen, KU Leuven, Belgium Jeroen Van Houdt, KU Leuven, Belgium Luc Dehaspe, UZ Leuven, Belgium Joshua A Suhl, Emory University Jurgen Del-Favero, University of Antwerp Geert Mortier, Antwerp University Hospital Elaine Zackai, The Children's Hospital of Philadelphia Ann Swillen, KU Leuven, Belgium Only first 10 authors above; see publication for full author list. Journal Title: Human Genome Variation Volume: Volume 3 Publisher: Nature Publishing Group: Open Access Journals - Option B | 2016-01-14, Pages 15065-15065 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.1038/hgv.2015.65 Permanent URL: https://pid.emory.edu/ark:/25593/rncxx Final published version: http://dx.doi.org/10.1038/hgv.2015.65 Copyright information: © 2016 Official journal of the Japan Society of Human Genetics This is an Open Access work distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Accessed September 28, 2021 7:41 PM EDT OPEN Citation: Human Genome Variation (2016) 3, 15065; doi:10.1038/hgv.2015.65 Official journal of the Japan Society of Human Genetics 2054-345X/16 www.nature.com/hgv ARTICLE A catalog of hemizygous variation in 127 22q11 deletion patients Matthew S Hestand1, Beata A Nowakowska1,2,Elfi Vergaelen1, Jeroen Van Houdt1,3, Luc Dehaspe3, Joshua A Suhl4, Jurgen Del-Favero5, Geert Mortier6, Elaine Zackai7,8, Ann Swillen1, Koenraad Devriendt1, Raquel E Gur8, Donna M McDonald-McGinn7,8, Stephen T Warren4, Beverly S Emanuel7,8 and Joris R Vermeesch1 The 22q11.2 deletion syndrome is the most common microdeletion disorder, with wide phenotypic variability. -

Downloadable Code for Forms the Data Analysis and Renders a Visual Display
Open Access Software2005KemmeretVolume al. 6, Issue 12, Article R106 Ulysses - an application for the projection of molecular interactions comment across species Danielle Kemmer*†, Yong Huang‡, Sohrab P Shah‡¥, Jonathan Lim†, Jochen Brumm†, Macaire MS Yuen‡, John Ling‡, Tao Xu‡, Wyeth W Wasserman†§ and BF Francis Ouellette‡§¶ * † Addresses: Center for Genomics and Bioinformatics, Karolinska Institutet, 171 77 Stockholm, Sweden. Centre for Molecular Medicine and reviews Therapeutics, University of British Columbia, Vancouver V5Z 4H4, BC, Canada. ‡UBC Bioinformatics Centre, University of British Columbia, Vancouver V6T 1Z4, BC, Canada. §Department of Medical Genetics, University of British Columbia, Vancouver, BC, Canada. ¶Michael Smith Laboratories, University of British Columbia, Vancouver V6T 1Z4, BC, Canada. ¥Department of Computer Science, University of British Columbia, Vancouver V6T 1Z4, BC, Canada. Correspondence: Wyeth W Wasserman. E-mail: [email protected] Published: 2 December 2005 Received: 23 February 2005 Revised: 3 August 2005 reports Genome Biology 2005, 6:R106 (doi:10.1186/gb-2005-6-12-r106) Accepted: 8 November 2005 The electronic version of this article is the complete one and can be found online at http://genomebiology.com/2005/6/12/R106 © 2005 Kemmer et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. deposited research Projecting<p>Ulysses, molecular a new software interactions for the across parallel species analysis and display of protein interactions detected in various species, is described.</p> Abstract We developed Ulysses as a user-oriented system that uses a process called Interolog Analysis for the parallel analysis and display of protein interactions detected in various species. -

Human Social Genomics in the Multi-Ethnic Study of Atherosclerosis
Getting “Under the Skin”: Human Social Genomics in the Multi-Ethnic Study of Atherosclerosis by Kristen Monét Brown A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Epidemiological Science) in the University of Michigan 2017 Doctoral Committee: Professor Ana V. Diez-Roux, Co-Chair, Drexel University Professor Sharon R. Kardia, Co-Chair Professor Bhramar Mukherjee Assistant Professor Belinda Needham Assistant Professor Jennifer A. Smith © Kristen Monét Brown, 2017 [email protected] ORCID iD: 0000-0002-9955-0568 Dedication I dedicate this dissertation to my grandmother, Gertrude Delores Hampton. Nanny, no one wanted to see me become “Dr. Brown” more than you. I know that you are standing over the bannister of heaven smiling and beaming with pride. I love you more than my words could ever fully express. ii Acknowledgements First, I give honor to God, who is the head of my life. Truly, without Him, none of this would be possible. Countless times throughout this doctoral journey I have relied my favorite scripture, “And we know that all things work together for good, to them that love God, to them who are called according to His purpose (Romans 8:28).” Secondly, I acknowledge my parents, James and Marilyn Brown. From an early age, you two instilled in me the value of education and have been my biggest cheerleaders throughout my entire life. I thank you for your unconditional love, encouragement, sacrifices, and support. I would not be here today without you. I truly thank God that out of the all of the people in the world that He could have chosen to be my parents, that He chose the two of you. -

Spliceosomal Genes in the D. Discoideum Genome: a Comparison with Those in H
Protein Cell 2011, 2(5): 395–409 Protein & Cell DOI 10.1007/s13238-011-1052-z RESEARCH ARTICLE Spliceosomal genes in the D. discoideum genome: a comparison with those in H. sapiens, D. melanogaster, A. thaliana and S. cerevisiae 1 2 2 4 4 2 Bing Yu , Petra✉ Fey , Karen E. Kestin-Pilcher , Alexei Fedorov , Ashwin Prakash , Rex L. Chisholm , Jane Y. Wu3 1 Department of Molecular and Clinical Genetics, Royal Prince Alfred Hospital and Sydney Medical School (Central), the University of Sydney, NSW 2006, Australia 2 dictyBase, Center for Genetic Medicine, Northwestern University, Chicago, IL 60611, USA 3 Department of Neurology and Lurie Comprehensive Cancer Center, Center for Genetic Medicine, Northwestern University Feinberg Medical School, Chicago, IL 60611, USA 4 Department of Medicine and Program in Bioinformatics and Proteomics/Genomics, The University of Toledo, Toledo, OH 43614, USA ✉ Correspondence: [email protected] Received May 11, 2011 Accepted May 20, 2011 ABSTRACT KEYWORDS pre-mRNA splicing, spliceosomal genes, Dictyostelium discoideum, comparative genomics, splicing Little is known about pre-mRNA splicing in Dictyostelium regulators discoideum although its genome has been completely sequenced. Our analysis suggests that pre-mRNA spli- INTRODUCTION cing plays an important role in D. discoideum gene expression as two thirds of its genes contain at least one The amoeboid protozoan Dictyostelium discoideum is a intron. Ongoing curation of the genome to date has eukaryotic model organism that has been extensively used revealed 40 genes in D. discoideum with clear evidence in studying signal transduction, cell motility and cell differ- of alternative splicing, supporting the existence of entiation. -

Examples of the Complex Architecture of the Human Transcriptome Revealed by RACE and High-Density Tiling Arrays
Downloaded from genome.cshlp.org on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press Methods Examples of the complex architecture of the human transcriptome revealed by RACE and high-density tiling arrays Philipp Kapranov,1 Jorg Drenkow, Jill Cheng, Jeffrey Long, Gregg Helt, Sujit Dike, and Thomas R. Gingeras Affymetrix Inc., Santa Clara, California 95051, USA Recently, we mapped the sites of transcription across ∼30% of the human genome and elucidated the structures of several hundred novel transcripts. In this report, we describe a novel combination of techniques including the rapid amplification of cDNA ends (RACE) and tiling array technologies that was used to further characterize transcripts in the human transcriptome. This technical approach allows for several important pieces of information to be gathered about each array-detected transcribed region, including strand of origin, start and termination positions, and the exonic structures of spliced and unspliced coding and noncoding RNAs. In this report, the structures of transcripts from 14 transcribed loci, representing both known genes and unannotated transcripts taken from the several hundred randomly selected unannotated transcripts described in our previous work are represented as examples of the complex organization of the human transcriptome. As a consequence of this complexity, it is not unusual that a single base pair can be part of an intricate network of multiple isoforms of overlapping sense and antisense transcripts, the majority of which are unannotated. Some of these transcripts follow the canonical splicing rules, whereas others combine the exons of different genes or represent other types of noncanonical transcripts. These results have important implications concerning the correlation of genotypes to phenotypes, the regulation of complex interlaced transcriptional patterns, and the definition of a gene. -

Comparative Genomics and Novel Bioinformatics Methodology
Comparative Genomics and Novel Bioinformatics Methodology Applied to the Green Anole Reveal Unique Sex Chromosome Evolution by Shawn Michael Rupp A Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science Approved June 2016 by the Graduate Supervisory Committee: Melissa Wilson Sayres, Chair Dale DeNardo Kenro Kusumi ARIZONA STATE UNIVERSITY December 2016 ABSTRACT In species with highly heteromorphic sex chromosomes, the degradation of one of the sex chromosomes can result in unequal gene expression between the sexes (e.g., between XX females and XY males) and between the sex chromosomes and the autosomes. Dosage compensation is a process whereby genes on the sex chromosomes achieve equal gene expression which prevents deleterious side effects from having too much or too little expression of genes on sex chromsomes. The green anole is part of a group of species that recently underwent an adaptive radiation. The green anole has XX/XY sex determination, but the content of the X chromosome and its evolution have not been described. Given its status as a model species, better understanding the green anole genome could reveal insights into other species. Genomic analyses are crucial for a comprehensive picture of sex chromosome differentiation and dosage compensation, in addition to understanding speciation. In order to address this, multiple comparative genomics and bioinformatics analyses were conducted to elucidate patterns of evolution in the green anole and across multiple anole species. Comparative genomics analyses were used to infer additional X-linked loci in the green anole, RNAseq data from male and female samples were anayzed to quantify patterns of sex-biased gene expression across the genome, and the extent of dosage compensation on the anole X chromosome was characterized, providing evidence that the sex chromosomes in the green anole are dosage compensated.