The Effect of Visual Capacity and Swimming Ability of Fish on the Performance of Light-Based Bycatch Reduction Devices in Prawn Trawls
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FISH LIST WISH LIST: a Case for Updating the Canadian Government’S Guidance for Common Names on Seafood
FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood Authors: Christina Callegari, Scott Wallace, Sarah Foster and Liane Arness ISBN: 978-1-988424-60-6 © SeaChoice November 2020 TABLE OF CONTENTS GLOSSARY . 3 EXECUTIVE SUMMARY . 4 Findings . 5 Recommendations . 6 INTRODUCTION . 7 APPROACH . 8 Identification of Canadian-caught species . 9 Data processing . 9 REPORT STRUCTURE . 10 SECTION A: COMMON AND OVERLAPPING NAMES . 10 Introduction . 10 Methodology . 10 Results . 11 Snapper/rockfish/Pacific snapper/rosefish/redfish . 12 Sole/flounder . 14 Shrimp/prawn . 15 Shark/dogfish . 15 Why it matters . 15 Recommendations . 16 SECTION B: CANADIAN-CAUGHT SPECIES OF HIGHEST CONCERN . 17 Introduction . 17 Methodology . 18 Results . 20 Commonly mislabelled species . 20 Species with sustainability concerns . 21 Species linked to human health concerns . 23 Species listed under the U .S . Seafood Import Monitoring Program . 25 Combined impact assessment . 26 Why it matters . 28 Recommendations . 28 SECTION C: MISSING SPECIES, MISSING ENGLISH AND FRENCH COMMON NAMES AND GENUS-LEVEL ENTRIES . 31 Introduction . 31 Missing species and outdated scientific names . 31 Scientific names without English or French CFIA common names . 32 Genus-level entries . 33 Why it matters . 34 Recommendations . 34 CONCLUSION . 35 REFERENCES . 36 APPENDIX . 39 Appendix A . 39 Appendix B . 39 FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood 2 GLOSSARY The terms below are defined to aid in comprehension of this report. Common name — Although species are given a standard Scientific name — The taxonomic (Latin) name for a species. common name that is readily used by the scientific In nomenclature, every scientific name consists of two parts, community, industry has adopted other widely used names the genus and the specific epithet, which is used to identify for species sold in the marketplace. -

Do Some Atlantic Bluefin Tuna Skip Spawning?
SCRS/2006/088 Col. Vol. Sci. Pap. ICCAT, 60(4): 1141-1153 (2007) DO SOME ATLANTIC BLUEFIN TUNA SKIP SPAWNING? David H. Secor1 SUMMARY During the spawning season for Atlantic bluefin tuna, some adults occur outside known spawning centers, suggesting either unknown spawning regions, or fundamental errors in our current understanding of bluefin tuna reproductive schedules. Based upon recent scientific perspectives, skipped spawning (delayed maturation and non-annual spawning) is possibly prevalent in moderately long-lived marine species like bluefin tuna. In principle, skipped spawning represents a trade-off between current and future reproduction. By foregoing reproduction, an individual can incur survival and growth benefits that accrue in deferred reproduction. Across a range of species, skipped reproduction was positively correlated with longevity, but for non-sturgeon species, adults spawned at intervals at least once every two years. A range of types of skipped spawning (constant, younger, older, event skipping; and delays in first maturation) was modeled for the western Atlantic bluefin tuna population to test for their effects on the egg-production-per-recruit biological reference point (stipulated at 20% and 40%). With the exception of extreme delays in maturation, skipped spawning had relatively small effect in depressing fishing mortality (F) threshold values. This was particularly true in comparison to scenarios of a juvenile fishery (ages 4-7), which substantially depressed threshold F values. Indeed, recent F estimates for 1990-2002 western Atlantic bluefin tuna stock assessments were in excess of threshold F values when juvenile size classes were exploited. If western bluefin tuna are currently maturing at an older age than is currently assessed (i.e., 10 v. -

NOAA's Description of the U.S Commercial Fisheries Including The
6.0 DESCRIPTION OF THE PELAGIC LONGLINE FISHERY FOR ATLANTIC HMS The HMS FMP provides a thorough description of the U.S. fisheries for Atlantic HMS, including sectors of the pelagic longline fishery. Below is specific information regarding the catch of pelagic longline fishermen in the Gulf of Mexico and off the Southeast coast of the United States. For more detailed information on the fishery, please refer to the HMS FMP. 6.1 Pelagic Longline Gear The U.S. pelagic longline fishery for Atlantic HMS primarily targets swordfish, yellowfin tuna, or bigeye tuna in various areas and seasons. Secondary target species include dolphin, albacore tuna, pelagic sharks including mako, thresher, and porbeagle sharks, as well as several species of large coastal sharks. Although this gear can be modified (i.e., depth of set, hook type, etc.) to target either swordfish, tunas, or sharks, like other hook and line fisheries, it is a multispecies fishery. These fisheries are opportunistic, switching gear style and making subtle changes to the fishing configuration to target the best available economic opportunity of each individual trip. Longline gear sometimes attracts and hooks non-target finfish with no commercial value, as well as species that cannot be retained by commercial fishermen, such as billfish. Pelagic longline gear is composed of several parts. See Figure 6.1. Figure 6.1. Typical U.S. pelagic longline gear. Source: Arocha, 1997. When targeting swordfish, the lines generally are deployed at sunset and hauled in at sunrise to take advantage of the nocturnal near-surface feeding habits of swordfish. In general, longlines targeting tunas are set in the morning, deeper in the water column, and hauled in the evening. -

Food Preference of Penaeus Vannamei
Gulf and Caribbean Research Volume 8 Issue 3 January 1991 Food Preference of Penaeus vannamei John T. Ogle Gulf Coast Research Laboratory Kathy Beaugez Gulf Coast Research Laboratory Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Ogle, J. T. and K. Beaugez. 1991. Food Preference of Penaeus vannamei. Gulf Research Reports 8 (3): 291-294. Retrieved from https://aquila.usm.edu/gcr/vol8/iss3/9 DOI: https://doi.org/10.18785/grr.0803.09 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf Research Reports, Vol. 8, No. 3, 291-294, 1991 FOOD PREFERENCE OF PENAEUS VANNAMEZ JOHN T. OGLE AND KATHY BEAUGEZ Fisheries Section, Guy Coast Research Laboratory, P.O. Box 7000, Ocean Springs, Mississippi 39464 ABSTRACT The preference of Penaeus vannamei for 15 food items used in maturation was determined. The foods in order of preference were ranked as follows: Artemia, krill, Maine bloodworms, oysters, sandworms, anchovies, Panama bloodworms, Nippai maturation pellets, Shigueno maturation pellets, conch, squid, Salmon-Frippak maturation pellets, Rangen maturation pellets and Argent maturation pellets. INTRODUCTION preference, but may have been chosen due to the stability and density of the pellets. Hardin (1981), working with There is a paucity of published prawn preference P. stylirostris, noted that one marine ration which in- papers. Studies have been undertaken to determine the cluded fish meal was preferred over another artificial distribution of potential prey from the natural shrimp diet made with soybean. -

Brown Tiger Prawn (Penaeus Esculentus)
I & I NSW WILD FISHERIES RESEARCH PROGRAM Brown Tiger Prawn (Penaeus esculentus) EXPLOITATION STATUS UNDEFINED NSW is at the southern end of the species’ range. Recruitment is likely to be small and variable. SCIENTIFIC NAME COMMON NAME COMMENT Penaeus esculentus brown tiger prawn Native to NSW waters Also known as leader prawn and giant Penaeus monodon black tiger prawn tiger prawn - farmed in NSW. Penaeus esculentus Image © Bernard Yau Background There are a number of large striped ‘tiger’ prawns waters in mud, sand or silt substrates less than known from Australian waters. Species such as 30 m deep. Off northern Australia, female brown the black tiger prawn (Penaeus monodon) and tiger prawns mature between 2.5 and 3.5 cm grooved tiger prawn (P. semisulcatus) have wide carapace length (CL) and grow to a maximum of tropical distributions throughout the Indo-West about 5.5 cm CL; males grow to a maximum of Pacific and northern Australia. The brown tiger about 4 cm CL. Spawning occurs mainly in water prawn (P. esculentus) is also mainly tropical but temperatures around 28-30°C, and the resulting appears to be endemic to Australia, inhabiting planktonic larvae are dispersed by coastal shallow coastal waters and estuaries from currents back into the estuaries to settle. central NSW (Sydney), around the north of the continent, to Shark Bay in WA. This species is Compared to northern Australian states, the fished commercially throughout its range and NSW tiger prawn catch is extremely small. Since contributes almost 30% of the ~1800 t tiger 2000, reported landings have been between prawn fishery (70% grooved tiger prawn) in the 3 and 6 t per year, with about half taken Northern Prawn Fishery of northern Australia. -

Capture-Based Aquaculture of Mullets in Egypt
109 Capture-based aquaculture of mullets in Egypt Magdy Saleh General Authority for Fish Resources Development Cairo, Egypt E-mail: [email protected] Saleh, M. 2008. Capture-based aquaculture of mullets in Egypt. In A. Lovatelli and P.F. Holthus (eds). Capture-based aquaculture. Global overview. FAO Fisheries Technical Paper. No. 508. Rome, FAO. pp. 109–126. SUMMARY The use of wild-caught mullet seed for the annual restocking of inland lakes has been known in Egypt for more than eight decades. The importance of wild seed collection increased with recent aquaculture developments. The positive experience with wild seed collection and high seed production costs has prevented the development of commercial mullet hatcheries. Mullet are considered very important aquaculture fish in Egypt with 156 400 tonnes produced in 2005 representing 29 percent of the national aquaculture production. Current legislation prohibits wild seed fisheries except under the direct supervision of the relevant authorities. In 2005, 69.4 million mullet fry were caught for both aquaculture and culture-based fisheries. A parallel illegal fishery exists, undermining proper management of the resources. The effect of wild seed fisheries on the wild stocks of mullet is not well studied. The negative effect of the activity is a matter of debate between fish farming and capture fisheries communities. Data on the capture of wild mullet fisheries shows no observable effect of fry collection on the catch during the last 25 years. DESCRIPTION OF THE SPECIES AND USE IN AQUACULTURE Species presentation Mullets are members of the Order Mugiliformes, Family Mugilidae. Mullets are ray- finned fish found worldwide in coastal temperate and tropical waters and, for some species, also in freshwater. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

Download Full Article 1.0MB .Pdf File
Memoirs of the Museum of Victoria 57( I): 143-165 ( 1998) 1 May 1998 https://doi.org/10.24199/j.mmv.1998.57.08 FISHES OF WILSONS PROMONTORY AND CORNER INLET, VICTORIA: COMPOSITION AND BIOGEOGRAPHIC AFFINITIES M. L. TURNER' AND M. D. NORMAN2 'Great Barrier Reef Marine Park Authority, PO Box 1379,Townsville, Qld 4810, Australia ([email protected]) 1Department of Zoology, University of Melbourne, Parkville, Vic. 3052, Australia (corresponding author: [email protected]) Abstract Turner, M.L. and Norman, M.D., 1998. Fishes of Wilsons Promontory and Comer Inlet. Victoria: composition and biogeographic affinities. Memoirs of the Museum of Victoria 57: 143-165. A diving survey of shallow-water marine fishes, primarily benthic reef fishes, was under taken around Wilsons Promontory and in Comer Inlet in 1987 and 1988. Shallow subtidal reefs in these regions are dominated by labrids, particularly Bluethroat Wrasse (Notolabrus tet ricus) and Saddled Wrasse (Notolabrus fucicola), the odacid Herring Cale (Odax cyanomelas), the serranid Barber Perch (Caesioperca rasor) and two scorpidid species, Sea Sweep (Scorpis aequipinnis) and Silver Sweep (Scorpis lineolata). Distributions and relative abundances (qualitative) are presented for 76 species at 26 sites in the region. The findings of this survey were supplemented with data from other surveys and sources to generate a checklist for fishes in the coastal waters of Wilsons Promontory and Comer Inlet. 23 I fishspecies of 92 families were identified to species level. An additional four species were only identified to higher taxonomic levels. These fishes were recorded from a range of habitat types, from freshwater streams to marine habitats (to 50 m deep). -
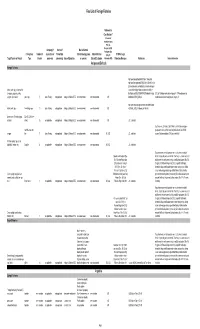
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Targeted Review of Biological and Ecological Information from Fisheries Research in the South East Marine Region
TARGETED REVIEW OF BIOLOGICAL AND ECOLOGICAL INFORMATION FROM FISHERIES RESEARCH IN THE SOUTH EAST MARINE REGION FINAL REPORT B. D. Bruce, R. Bradford, R. Daley, M. Green and K. Phillips December 2002 Client: National Oceans Office Targeted review of biological and ecological information from fisheries research in the South East Marine Region Final Report B. D. Bruce, R. Bradford, R. Daley M. Green and K. Phillips* CSIRO Marine Research, Hobart * National Oceans Office December 2002 2 Table of Contents: Table of Contents:...................................................................................................................................3 Introduction.............................................................................................................................................5 Objective of review.............................................................................................................................5 Structure of review..............................................................................................................................5 Format.................................................................................................................................................6 General ecological/biological issues and uncertainties for the South East Marine Region ....................9 Specific fishery and key species accounts ............................................................................................10 South East Fishery (SEF) including the South East Trawl -

The Diversity and Adaptive Evolution of Visual Photopigments in Reptiles Frontiers in Ecology and Evolution, 7: 352
http://www.diva-portal.org This is the published version of a paper published in Frontiers in Ecology and Evolution. Citation for the original published paper (version of record): Katti, C., Stacey-Solis, M., Anahí Coronel-Rojas, N., Davies, W I. (2019) The Diversity and Adaptive Evolution of Visual Photopigments in Reptiles Frontiers in Ecology and Evolution, 7: 352 https://doi.org/10.3389/fevo.2019.00352 Access to the published version may require subscription. N.B. When citing this work, cite the original published paper. Permanent link to this version: http://urn.kb.se/resolve?urn=urn:nbn:se:umu:diva-164181 REVIEW published: 19 September 2019 doi: 10.3389/fevo.2019.00352 The Diversity and Adaptive Evolution of Visual Photopigments in Reptiles Christiana Katti 1*, Micaela Stacey-Solis 1, Nicole Anahí Coronel-Rojas 1 and Wayne Iwan Lee Davies 2,3,4,5,6 1 Escuela de Ciencias Biológicas, Pontificia Universidad Católica del Ecuador, Quito, Ecuador, 2 Center for Molecular Medicine, Umeå University, Umeå, Sweden, 3 Oceans Graduate School, University of Western Australia, Crawley, WA, Australia, 4 Oceans Institute, University of Western Australia, Crawley, WA, Australia, 5 School of Biological Sciences, University of Western Australia, Perth, WA, Australia, 6 Center for Ophthalmology and Visual Science, Lions Eye Institute, University of Western Australia, Perth, WA, Australia Reptiles are a highly diverse class that consists of snakes, geckos, iguanid lizards, and chameleons among others. Given their unique phylogenetic position in relation to both birds and mammals, reptiles are interesting animal models with which to decipher the evolution of vertebrate photopigments (opsin protein plus a light-sensitive retinal chromophore) and their contribution to vision.