Do Some Atlantic Bluefin Tuna Skip Spawning?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Educators' Resource Guide
EDUCATORS' RESOURCE GUIDE Produced and published by 3D Entertainment Distribution Written by Dr. Elisabeth Mantello In collaboration with Jean-Michel Cousteau’s Ocean Futures Society TABLE OF CONTENTS TO EDUCATORS .................................................................................................p 3 III. PART 3. ACTIVITIES FOR STUDENTS INTRODUCTION .................................................................................................p 4 ACTIVITY 1. DO YOU Know ME? ................................................................. p 20 PLANKton, SOURCE OF LIFE .....................................................................p 4 ACTIVITY 2. discoVER THE ANIMALS OF "SECRET OCEAN" ......... p 21-24 ACTIVITY 3. A. SECRET OCEAN word FIND ......................................... p 25 PART 1. SCENES FROM "SECRET OCEAN" ACTIVITY 3. B. ADD color to THE octoPUS! .................................... p 25 1. CHristmas TREE WORMS .........................................................................p 5 ACTIVITY 4. A. WHERE IS MY MOUTH? ..................................................... p 26 2. GIANT BasKET Star ..................................................................................p 6 ACTIVITY 4. B. WHat DO I USE to eat? .................................................. p 26 3. SEA ANEMONE AND Clown FISH ......................................................p 6 ACTIVITY 5. A. WHO eats WHat? .............................................................. p 27 4. GIANT CLAM AND ZOOXANTHELLAE ................................................p -

American Lobster Settlement Index |Update 2019
American Lobster Settlement Index | Update 2019 Compiled by: R. Wahle and K. Holmes Participants: ME DMR (K. Reardon, R. Russell), MA DMF (T. Pugh, K. Whitmore), C. Brown (Ready Seafood Co.), J. Drouin (Little River Lobster Co.), RI DFW (S. Olszewski, C. McManus), NH F&G (J. Carloni), DFO Canada (A. Rondeau, N. Asselin, J. Gaudette, P. Lawton, S. Armsworthy, A. Cook), UNB, St. John (R. Rochette), PEIFA (L. Ramsay, M. Giffen), PEI DAF (R. MacMillan), Fishermen & Scientists Research Society (S. Scott-Tibbets), Memorial University (A. Le Bris), http://umaine.edu/wahlelab/current-projects/american-lobster-settlement-index/ Several months now into the COVID-19 pandemic, we can only look back to the 2019 fishing year with some nostalgia. In calendar year 2019, Canadian lobster landings continued to boast near all-time highs. And while US landings have slipped a bit from their own historic highs, much of those losses have been offset by continued high value. That is, until now. All that changed almost overnight with the onset of the pandemic, as demand for lobster plummeted with shuttered restaurants and overseas commerce, dragging down the price of lobster, much to the distress of fishing communities up and down the coast. As we enter the 2020 summer fishing season, the lobster industry grapples to find a new normal as it accommodates health protocols, readjusts markets and scales back revenue expectations. In kind, state and federal marine resource monitoring programs are equally rethinking sampling programs under shrinking tax revenues and new safety standards. Last year’s Update examined how well ALSI predicted trends in the fishery from Fundy to Rhode Island. -

Characterization of Effluent from an Inland, Low- Salinity Shrimp Farm
Aquacultural Engineering 27 (2003) 147Á/156 www.elsevier.com/locate/aqua-online Characterization of effluent from an inland, low- salinity shrimp farm: what contribution could this water make if used for irrigation Dennis McIntosh ,Kevin Fitzsimmons Environmental Research Lab, University of Arizona, 2601 East Airport Drive, Tucson, AZ 85706, USA Received 7 July 2002; accepted 7 October 2002 Abstract Coastal aquaculture can contribute to eutrophication of receiving waters. New technologies and improved management practices allow the aquaculture industry to be more sustainable and economically viable. Current practices, however, do not provide an additional use for effluent water. Nitrogen, phosphorus and other effluent compounds could be valuable plant nutrients. Inflow and effluent water from an inland, low-salinity shrimp farm, were monitored. Bi-weekly analysis included total nitrogen, ammonia-nitrogen, nitrite-nitrogen, nitrate- nitrogen, total phosphorus, reactive phosphorus, alkalinity, chemical oxygen demand (COD), biochemical oxygen demand (BOD), total suspended solids (TSS) and volatile suspended solids (VSS), as well as temperature, salinity, dissolved oxygen and pH. Alkalinity and total nitrogen decreased during the in-pond residency. The other parameters increased while in the ponds. The potential benefit of having nutrient enriched wastewater to irrigate field crops was substantial, supplying between 20 and 31% of the necessary nitrogen fertilizer for wheat production. # 2002 Elsevier Science B.V. All rights reserved. Keywords: Inland aquaculture; Integration; Arizona; Marine shrimp Corresponding author. Tel.: /1-520-626-3318; fax: /1-520-573-0852 E-mail address: [email protected] (D. McIntosh). 0144-8609/02/$ - see front matter # 2002 Elsevier Science B.V. All rights reserved. PII: S 0 1 4 4 - 8 6 0 9 ( 0 2 ) 0 0 0 5 4 - 7 148 D. -

FISH LIST WISH LIST: a Case for Updating the Canadian Government’S Guidance for Common Names on Seafood
FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood Authors: Christina Callegari, Scott Wallace, Sarah Foster and Liane Arness ISBN: 978-1-988424-60-6 © SeaChoice November 2020 TABLE OF CONTENTS GLOSSARY . 3 EXECUTIVE SUMMARY . 4 Findings . 5 Recommendations . 6 INTRODUCTION . 7 APPROACH . 8 Identification of Canadian-caught species . 9 Data processing . 9 REPORT STRUCTURE . 10 SECTION A: COMMON AND OVERLAPPING NAMES . 10 Introduction . 10 Methodology . 10 Results . 11 Snapper/rockfish/Pacific snapper/rosefish/redfish . 12 Sole/flounder . 14 Shrimp/prawn . 15 Shark/dogfish . 15 Why it matters . 15 Recommendations . 16 SECTION B: CANADIAN-CAUGHT SPECIES OF HIGHEST CONCERN . 17 Introduction . 17 Methodology . 18 Results . 20 Commonly mislabelled species . 20 Species with sustainability concerns . 21 Species linked to human health concerns . 23 Species listed under the U .S . Seafood Import Monitoring Program . 25 Combined impact assessment . 26 Why it matters . 28 Recommendations . 28 SECTION C: MISSING SPECIES, MISSING ENGLISH AND FRENCH COMMON NAMES AND GENUS-LEVEL ENTRIES . 31 Introduction . 31 Missing species and outdated scientific names . 31 Scientific names without English or French CFIA common names . 32 Genus-level entries . 33 Why it matters . 34 Recommendations . 34 CONCLUSION . 35 REFERENCES . 36 APPENDIX . 39 Appendix A . 39 Appendix B . 39 FISH LIST WISH LIST: A case for updating the Canadian government’s guidance for common names on seafood 2 GLOSSARY The terms below are defined to aid in comprehension of this report. Common name — Although species are given a standard Scientific name — The taxonomic (Latin) name for a species. common name that is readily used by the scientific In nomenclature, every scientific name consists of two parts, community, industry has adopted other widely used names the genus and the specific epithet, which is used to identify for species sold in the marketplace. -

Cod Fact Sheet
R FACT SHEET Cod (Gadus morhua) Image taken from (Cohen et al. 1990) Introduction Cod (Gadus morhua) is generally considered a demersal fish although its habitat may become pelagic under certain hydrographic conditions, when feeding or spawning. It is widely distributed throughout the north Atlantic and Arctic regions in a variety of habitats from shoreline to continental shelf, in depths to 600m (Cohen et al. 1990). The Irish Sea stock spawns at two main sites in the western and eastern Irish Sea during February to April (Armstrong et al. 2011). Historically the stock has been commercially important, however in the last decade a decline in SSB and reduced productivity of the stock have led to reduce landings. Reviewed distribution map for Gadus morhua (Atlantic cod), with modelled year 2100 native range map based on IPCC A2 emissions scenario. www.aquamaps.org, version of Aug. 2013. Web. Accessed 28 Jan. 2011 Life history overview Adults are usually found in deeper, colder waters. During the day they form schools and swim about 30-80 m above the bottom, dispersing at night to feed (Cohen et al. 1990; ICES 2005). They are omnivorous; feeding at dawn or dusk on invertebrates and fish, including their own young (Cohen et al. 1990). Adults migrate between spawning, feeding and overwintering areas, mostly within the boundaries of the respective stocks. Large migrations are rare occurrences, although there is evidence for limited seasonal migrations into neighbouring regions, most Irish Sea fish will stay within their management area (ICES 2012). Historical tagging studies indicated spawning site fidelity but with varying degrees of mixing of cod between the Irish Sea, Celtic Sea and west of Scotland/north of Ireland (ICES 2015). -

American Eel (Anguilla Rostrata)
Indiana Division of Fish and Wildlife’s Animal Information Series American Eel (Anguilla rostrata) Do they have any other names? The names “glass eel” or “elver” are used to describe young, developing eels. Why are they called American eels? The closest relatives to the American eel are other freshwater eels found in Europe and Asia; therefore they are called American eels because they are only in America. Anguilla is the Latin name for eel and rostrata is Latin for “beaked,” in reference to the snout. What do they look like? American eels are a brownish-colored fish with a slender, snake-like body and a small, pointed head. The body appears smooth and mucousy; however there are small scales present. They have a long dorsal fin that is more than half the length of the body and attached to the tail and anal fins. American eels do not have pelvic fins, but do have pectoral fins (on the sides near the head). The lower jaw projects farther than the upper jaw and they have many small teeth. Photo Credit: Duane Raver/USFWS 2012-MLC Page 1 Where do they live in Indiana? American eels are rare in Indiana and are found in large streams and rivers. They can sometimes be found in ponds or lakes that are not connected to a river, although this seldom happens. What kind of habitat do they need? American eels are found in large streams or rivers with continuous flow and clear water. During the day eels like to stay near logs, boulders, or other cover. -

Atlantic Bluefin Tuna (Thunnus Thynnus) Population Dynamics
Environ. Sci. Technol. 2009, 43, 8522–8527 nations of the International Commission for the Conservation Atlantic Bluefin Tuna (Thunnus of Atlantic Tunas (ICCAT) currently manage ABFT fisheries thynnus) Population Dynamics assuming two units (a western stock spawning in the Gulf of Mexico, and an eastern stock which spawns in the Delineated by Organochlorine Mediterranean Sea) ostensibly separated by the 45° W meridian with little intermixing between stocks. However, Tracers tagging studies indicate that bluefin tuna undergo extensive and complex migrations, including trans-Atlantic migrations, ,† and that stock mixing could be as high as 30% (2-4). Extensive REBECCA M. DICKHUT,* - ASHOK D. DESHPANDE,‡ mixing of eastern and western stocks (35 57% bluefin tuna ALESSANDRA CINCINELLI,§ of eastern origin) within the U.S. Mid Atlantic Bight was also 18 MICHELE A. COCHRAN,† reported recently based on otolith δ O values (5). The SIMONETTA CORSOLINI,| uncertainty of stock structures due to mixing makes it difficult RICHARD W. BRILL,† DAVID H. SECOR,⊥ for fisheries managers to assess the effectiveness of rebuilding AND JOHN E. GRAVES† efforts for the dwindling western Atlantic spawning stock of Virginia Institute of Marine Science, Gloucester Point, bluefin tuna. Understanding ABFT spatial distributions and Virginia 23062, National Marine Fisheries Service, dynamics are vital for robust population assessments and Highlands, New Jersey 07732, Department of Chemistry, the design of effective management strategies, and there is University of Florence, 50019 -

Our Nation's Fisheries Will Be a Lot Easier Once We've Used up Everything Except Jellyfish!
Mismanaging Our Nation’s Fisheriesa menu of what's missing Limited quantity: get ‘em while supplies last Ted Stevens Alaskan Surprise Due to years of overfishing, we probably won’t be serving up Pacific Ocean perch, Tanner crab, Greenland turbot or rougheye rockfish. They may be a little hard to swallow, but Senator Stevens and the North Pacific Council will be sure to offer last minute riders, father and son sweetheart deals, record-breaking quotas, industry-led research, conflicts of interest and anti-trust violations. Meanwhile, fur seals, sea lions and sea otters are going hungry and disappearing fast. Surprise! Pacific Rockfish: See No Fish, Eat No Fish Cowcod, Canary Rockfish and Bocaccio are just three examples of rockfish managed by the Pacific Council that are overfished. As for the exact number of West Coast groundfish that are overfished, who knows? Without surveys to tell them what’s going on, what are they managing exactly? Striped Bass: Thin Is In! This popular Atlantic rockfish is available in abundance. Unfortunately, many appear to be undernourished and suffering from lesions – a condition that may point to Omega Protein’s industrial fishery of menhaden, the striper’s favorite prey species. Actually, you may want to hold off on this one until ASMFC starts regulating menhaden. Can you believe there are still no catch limits? Red Snapper Bycatch Platter While we are unable to provide full-size red snapper, we offer this plate of twenty juvenile red snapper discarded as bycatch from a Gulf of Mexico shrimp trawler for your dining pleasure. Shrimpers take and throw away about half of all young red snappers along the Texas coast, so we’ll keep these little guys coming straight from the back of the boat to the back of your throat! Caribbean Reef Fish Grab Bag What’s for dinner from the Caribbean? Who knows? With coral reefs in their jurisdiction, you would expect the Caribbean Council to be pioneering the ecosystem-based management approach and implementing the precautionary principle approach. -

NOAA's Description of the U.S Commercial Fisheries Including The
6.0 DESCRIPTION OF THE PELAGIC LONGLINE FISHERY FOR ATLANTIC HMS The HMS FMP provides a thorough description of the U.S. fisheries for Atlantic HMS, including sectors of the pelagic longline fishery. Below is specific information regarding the catch of pelagic longline fishermen in the Gulf of Mexico and off the Southeast coast of the United States. For more detailed information on the fishery, please refer to the HMS FMP. 6.1 Pelagic Longline Gear The U.S. pelagic longline fishery for Atlantic HMS primarily targets swordfish, yellowfin tuna, or bigeye tuna in various areas and seasons. Secondary target species include dolphin, albacore tuna, pelagic sharks including mako, thresher, and porbeagle sharks, as well as several species of large coastal sharks. Although this gear can be modified (i.e., depth of set, hook type, etc.) to target either swordfish, tunas, or sharks, like other hook and line fisheries, it is a multispecies fishery. These fisheries are opportunistic, switching gear style and making subtle changes to the fishing configuration to target the best available economic opportunity of each individual trip. Longline gear sometimes attracts and hooks non-target finfish with no commercial value, as well as species that cannot be retained by commercial fishermen, such as billfish. Pelagic longline gear is composed of several parts. See Figure 6.1. Figure 6.1. Typical U.S. pelagic longline gear. Source: Arocha, 1997. When targeting swordfish, the lines generally are deployed at sunset and hauled in at sunrise to take advantage of the nocturnal near-surface feeding habits of swordfish. In general, longlines targeting tunas are set in the morning, deeper in the water column, and hauled in the evening. -

A Preliminary Study on the Stomach Content of Southern Bluefin Tuna Thunnus Maccoyii Caught by Taiwanese Longliner in the Central Indian Ocean
CCSBT-ESC/0509/35 A preliminary study on the stomach content of southern bluefin tuna Thunnus maccoyii caught by Taiwanese longliner in the central Indian Ocean Kwang-Ming Liu1, Wei-Ke Chen2, Shoou-Jeng Joung2, and Sui-Kai Chang3 1. Institute of Marine Resource Management, National Taiwan Ocean University, Keelung, Taiwan. 2. Department of Environmental Biology and Fisheries Science, National Taiwan Ocean University, Keelung, Taiwan. 3. Fisheries Agency, Council of Agriculture, Taipei, Taiwan. Abstract The stomach contents of 63 southern bluefin tuna captured by Taiwanese longliners in central Indian Ocean in August 2004 were examined. The size of tunas ranged from 84-187 cm FL (12-115 kg GG). The length and weight frequency distributions indicated that most specimens were in the range of 100-130 cm FL with a body weight between 10 and 30 kg for both sexes. The sexes- combined relationship between dressed weight and fork length can be described by W = 6.975× 10-6× FL3.1765 (n=56, r2=0.967, p < 0.05). The subjective index of fullness of specimens was estimated as: 1 = empty (38.6%), 2 = <half full (47.37%), 3 = half full (3.51%), 4 = >half full (5.26%), and 5 = full (5.26%). For the stomachs with prey items, almost all the preys are pisces and the proportion of each prey groups are fishes (95.6%), cephalopods (2.05%), and crustaceans (0.02%). In total, 6 prey taxa were identified – 4 species of fish, 1 unidentified pisces, 1 unidentified crustacean, and 1 unidentified squid. The 4 fish species fall in the family of Carangidae, Clupeidae, Emmelichthyidae, and Hemiramphidae. -

IATTC-94-01 the Tuna Fishery, Stocks, and Ecosystem in the Eastern
INTER-AMERICAN TROPICAL TUNA COMMISSION 94TH MEETING Bilbao, Spain 22-26 July 2019 DOCUMENT IATTC-94-01 REPORT ON THE TUNA FISHERY, STOCKS, AND ECOSYSTEM IN THE EASTERN PACIFIC OCEAN IN 2018 A. The fishery for tunas and billfishes in the eastern Pacific Ocean ....................................................... 3 B. Yellowfin tuna ................................................................................................................................... 50 C. Skipjack tuna ..................................................................................................................................... 58 D. Bigeye tuna ........................................................................................................................................ 64 E. Pacific bluefin tuna ............................................................................................................................ 72 F. Albacore tuna .................................................................................................................................... 76 G. Swordfish ........................................................................................................................................... 82 H. Blue marlin ........................................................................................................................................ 85 I. Striped marlin .................................................................................................................................... 86 J. Sailfish -
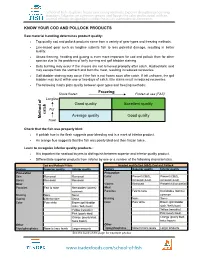
Know Your Cod and Pollock Products
School of Fish - Explores frozen processing methods, Exposes deceptive processing practices, Educates on common species and Equips the sales professional with an arsenal of tools designed to combat the issues vulnerable to deception. KNOW YOUR COD AND POLLOCK PRODUCTS Raw material handling determines product quality: • Top quality cod and pollock products come from a variety of gear types and freezing methods. • Line-based gear such as longline submits fish to less potential damage, resulting in better quality. • At-sea freezing, heading and gutting is even more important for cod and pollock than for other species due to the problems of belly burning and gall bladder staining. • Belly burning may occur if the viscera are not removed promptly after catch. Hydrochloric acid may escape from the stomach and burn the meat, resulting in reduced recoveries. • Gall bladder staining may occur if the fish is not frozen soon after catch. If left unfrozen, the gall bladder may burst within one or two days of catch. Bile stains result in reduced recoveries. • The following matrix plots quality between gear types and freezing methods: Shore frozen Freezing Frozen at sea (FAS) Longline of Good quality Excellent quality Pot catch Average quality Good quality Method Trawl Check that the fish was properly bled: • A pinkish hue in the flesh suggests poor bleeding and is a mark of inferior product. • An orange hue suggests that the fish was poorly bled and then frozen twice. Learn to recognize inferior quality products: • It is important for seafood buyers to distinguish between superior and inferior quality product. • Differentiate superior products from inferior by one or a number of the following characteristics.