Coprophilous Fungi from Iceland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Íbúakönnun Á Íslandi
ÍBÚAKÖNNUN Á ÍSLANDI Staða og mikilvægi búsetuskilyrða 19 landsvæða á landsbyggðunum frá Hornafirði í austri að Skagafirði í norðri Vífill Karlsson Samtök sveitarfélaga á Vesturlandi Skýrsla SSV Nr. 1 2018 Maí 2018 ISSN 1670-7923 EFNISYFIRLIT 1 Samantekt .................................................................................................................................................................... 8 2 Inngangur ................................................................................................................................................................. 10 3 Aðferð og gögn ........................................................................................................................................................ 10 3.1 Aðferðir í úrvinnslu innan hvers landshluta .................................................................................... 13 3.2 Aðferðir í samanburði á milli allra landshluta ................................................................................. 15 4 Niðurstöður íbúakannana á 19 landsvæðum ............................................................................................ 16 4.1 Almenn velferð íbúanna, ánægja þeirra og framtíðaráform ...................................................... 16 4.1.1 Aðferð: Meðaltöl og frávik afstöðu kvenna, ungra og þeirra sem búa í sveit ............ 16 4.1.2 Hamingja ................................................................................................................................................ 17 -

Studies of Coprophilous Ascomycetes in Kenya – Ascobolus Species from Wildlife Dung
Current Research in Environmental & Applied Mycology Doi 10.5943/cream/2/1/1 Studies of coprophilous ascomycetes in Kenya – Ascobolus species from wildlife dung Mungai PG1,2,3, Njogu JG3, Chukeatirote E1,2 and Hyde KD1,2* 1Institute of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 2School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 3Biodiversity Research and Monitoring Division, Kenya Wildlife Service, P.O. Box 40241 00100 Nairobi, Kenya Mungai PG, Njogu JG, Chukeatirote E, Hyde KD 2012 – Studies of coprophilous ascomycetes in Kenya – Ascobolus species from wildlife dung. Current Research in Environmental & Applied Mycology 2(1), 1-16, Doi 10.5943/cream/2/1/1 Species of coprophilous Ascobolus were examined in a study of coprophilous fungi in different habitats and wildlife dung types from National Parks in Kenya. Dung samples were collected in the field and returned to the laboratory where they were incubated in moist chamber culture. Coprophilous Ascobolus were isolated from giraffe, impala, common zebra, African elephant dung, Cape buffalo, dikdik, hippopotamus, black rhinoceros and waterbuck dung. Six species, Ascobolus amoenus, A. bistisii, A. calesco, A. immersus, A. nairobiensis and A. tsavoensis are identified and described. Ascobolus calesco, A. amoenus and A. bistisii were the most common. Two new species, Ascobolus nairobiensis and A. tsavoensis are introduced in this paper. In addition, two others, Ascobolus bistisii and A. calesco are new records in Kenya and are described and illustrated. The diversity of coprophilous Ascobolus from wildlife dung in Kenya as deduced from this study is very high. Key words – Ascobolus amoenus – A. -

A Taxonomic Study of the Coprophilous Ascomycetes Of
Eastern Illinois University The Keep Masters Theses Student Theses & Publications 1971 A Taxonomic Study of the Coprophilous Ascomycetes of Southeastern Illinois Alan Douglas Parker Eastern Illinois University This research is a product of the graduate program in Botany at Eastern Illinois University. Find out more about the program. Recommended Citation Parker, Alan Douglas, "A Taxonomic Study of the Coprophilous Ascomycetes of Southeastern Illinois" (1971). Masters Theses. 3958. https://thekeep.eiu.edu/theses/3958 This is brought to you for free and open access by the Student Theses & Publications at The Keep. It has been accepted for inclusion in Masters Theses by an authorized administrator of The Keep. For more information, please contact [email protected]. PA ER CERTIFICATE #2 f TO Graduate Degree Candidates who have written formal theses. SUBJECT: Permission to reproduce theses. Th' University Library is receiving a number of requests from other ins.litutions asking permission to reproduce dissertations for inclusion in their library holdings. Although no copyright laws are involved, we:feel that professional courtesy demands that permission be obtained frqm the author before we allow theses to be copied. Ple'ase sign one of the following statements. Bo9th Library of 'Eastern Illinois University has my permission to le�� my thesis to a reputable college or university for the purpose of copying it for inclusion in that institution's library or research hol�ings. Date Author I ��spectfully request Booth Library of Eastern Illinois University not al�pw my thesis be reproduced because � µ� 7 Date ILB1861.C57X P2381>C2/ A TAXONOMIC STUDY OF THE COPROPHILOUS ASCOMYCETES OF sou·rHEASTERN ILLINOIS (TITLE) BY ALAN DOUGLAS PARKER ...... -

Fungal Cannons: Explosive Spore Discharge in the Ascomycota Frances Trail
MINIREVIEW Fungal cannons: explosive spore discharge in the Ascomycota Frances Trail Department of Plant Biology and Department of Plant Pathology, Michigan State University, East Lansing, MI, USA Correspondence: Frances Trail, Department Abstract Downloaded from https://academic.oup.com/femsle/article/276/1/12/593867 by guest on 24 September 2021 of Plant Biology, Michigan State University, East Lansing, MI 48824, USA. Tel.: 11 517 The ascomycetous fungi produce prodigious amounts of spores through both 432 2939; fax: 11 517 353 1926; asexual and sexual reproduction. Their sexual spores (ascospores) develop within e-mail: [email protected] tubular sacs called asci that act as small water cannons and expel the spores into the air. Dispersal of spores by forcible discharge is important for dissemination of Received 15 June 2007; revised 28 July 2007; many fungal plant diseases and for the dispersal of many saprophytic fungi. The accepted 30 July 2007. mechanism has long been thought to be driven by turgor pressure within the First published online 3 September 2007. extending ascus; however, relatively little genetic and physiological work has been carried out on the mechanism. Recent studies have measured the pressures within DOI:10.1111/j.1574-6968.2007.00900.x the ascus and quantified the components of the ascus epiplasmic fluid that contribute to the osmotic potential. Few species have been examined in detail, Editor: Richard Staples but the results indicate diversity in ascus function that reflects ascus size, fruiting Keywords body type, and the niche of the particular species. ascus; ascospore; turgor pressure; perithecium; apothecium. 2 and 3). Each subphylum contains members that forcibly Introduction discharge their spores. -

Resurrection and Emendation of the Hypoxylaceae, Recognised from a Multigene Phylogeny of the Xylariales
Mycol Progress DOI 10.1007/s11557-017-1311-3 ORIGINAL ARTICLE Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales Lucile Wendt1,2 & Esteban Benjamin Sir3 & Eric Kuhnert1,2 & Simone Heitkämper1,2 & Christopher Lambert1,2 & Adriana I. Hladki3 & Andrea I. Romero4,5 & J. Jennifer Luangsa-ard6 & Prasert Srikitikulchai6 & Derek Peršoh7 & Marc Stadler1,2 Received: 21 February 2017 /Revised: 12 April 2017 /Accepted: 19 April 2017 # The Author(s) 2017. This article is an open access publication Abstract A multigene phylogeny was constructed, including polymerase II (RPB2), and beta-tubulin (TUB2). Specimens a significant number of representative species of the main were selected based on more than a decade of intensive mor- lineages in the Xylariaceae and four DNA loci the internal phological and chemotaxonomic work, and cautious taxon transcribed spacer region (ITS), the large subunit (LSU) of sampling was performed to cover the major lineages of the the nuclear rDNA, the second largest subunit of the RNA Xylariaceae; however, with emphasis on hypoxyloid species. The comprehensive phylogenetic analysis revealed a clear-cut This article is part of the “Special Issue on ascomycete systematics in segregation of the Xylariaceae into several major clades, honor of Richard P. Korf who died in August 2016”. which was well in accordance with previously established morphological and chemotaxonomic concepts. One of these The present paper is dedicated to Prof. Jack D. Rogers, on the occasion of his fortcoming 80th birthday. clades contained Annulohypoxylon, Hypoxylon, Daldinia,and other related genera that have stromatal pigments and a Section Editor: Teresa Iturriaga and Marc Stadler nodulisporium-like anamorph. -

Ascobolus Gomayapriya: a New Coprophilous Fungus from Andaman Islands, India
Studies in Fungi 3(1): 73–78 (2018) www.studiesinfungi.org ISSN 2465-4973 Article Doi 10.5943/sif/3/1/9 Copyright © Institute of Animal Science, Chinese Academy of Agricultural Sciences Ascobolus gomayapriya: A new coprophilous fungus from Andaman Islands, India Niranjan M and Sarma VV* Department of Biotechnology, Pondicherry University, Kalapet, Pondicherry-605014, India. Niranjan M, Sarma VV 2018 – Ascobolus gomayapriya: A new coprophilous fungus from Andaman Islands, India. Studies in Fungi 3(1), 73–78, Doi 10.5943/sif/3/1/9 Abstract Ascobolus is a very large genus among coprophilous fungi colonizing dung. There are very few workers who have explored dung fungi from India. During a recent trip to Andaman Islands, examination of cow dung samples revealed a new coprophilous fungus in the genus Ascobolus and the same has been reported in this paper. The present new species A. gomayapriya colonizes and grows on cow dung. A. gomayapriya is characterized by stalked, light-greenish-yellow apothecial ascomata, long cylindrical, short pedicellate asci with rounded apical caps, positive bluing reaction to Lougal’s reagent, ascospores that are hyaline to pale yellow red, smooth, cylindrical, thick- walled with two layers, sparsely dotted verruculose surface, very thin crevices. Key words – Ascomycetes – Dung fungi – Pezizomycetidae – Pezizales – Taxonomy Introduction Coprophilous fungi are an important part of the wildlife ecosystems as they help in recycling nutrients in animal dung in a saprophytic mode (Richardson 2001). Together with protozoa, myxomycetes, bacteria, nematodes and many insects, fungi are responsible for the breakdown of animal faeces, and for recycling the nutrients they contain. These are specialized fungi, able to withstand, and in many cases are dependent on, passage through an animal’s gut before growing on the dung (Richardson 2003). -
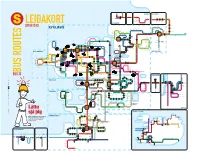
Strætó.Is Bus.Is
HÓLMAVÍK AKUREYRI REYKHOLT Melahverfi Akranes - Akratorg STYKKISHÓLMUR Akranes - Kirkjubraut Akranes - Þjóðbraut Borgarnes - N1 Akranes - Bæjarskrifstofur Kjalarnes - Kjalarnes - Kjalarnes - Akranes - Garðabraut Esjuskáli Esjugrund Klébergsskóli Akranes - Jaðarsbakki Akranes - Bresaflöt Esjurætur Skeljatangi Arnartangi STRÆTÓ.IS Þverholt Skei›holt Kelduskóli/Korpa Korpúlfssta›ir Bollatangi Brekkutangi Esjumelar Brúnasta›ir Bakkasta›ir Klapparhlí› FMOS Varmárskóli Leirvogstunga Skálatún Tröllaborgir Vættaborgir Jötnaborgir Strætóvegur Bar›asta›ir HÁHOLT v/Vættaborgir Mosavegur Goðaborgir Hulduborgir Borgarholtsskóli Kelduskóli/Vík Dofraborgir Go›aborgir Ásland Tjaldanes Mosfell Lundur Skólavegur Laufengi Egilshöll Borgavegur Dvergaborgir SPÖNG Jónsteigur Skarfagar›ar Kænugar›ar Borgavegur Borgavegur Úlfarsbraut SkyggnistorgSkyggnisbraut Úlfarsá Sundagar›ar Hé›insgata Hrafnista Hjallavegur Sægar›ar Víkurvegur Völuteigur Dalbraut Gullengi Mosarimi Lyngrimi Lambhagav./Mímisbrunnur Úlfarsbraut Fiskisló› Laugarnestangi Mi›gar›ur Keldnaholt Reykjalundur Lambhagav./Reynisvatnsvegur Fellsvegur Dælustö›varvegur Kirkjusandur Kirkjusandur Flétturimi Grunnsló› Höf›atorg Nóatún Hótel Cabin Borgartún Sund Holtagar›ar Hallsvegur Húsasmi›jan Reynisvatn Reykjabyggð LaugardalslaugLaugarásvegur Hrafnista Dragavegur Rimaflöt Gufunesbær Þúsöld Biskupsgata Hólsvegur Hólsvegur Dalhús Vallarhús BrekkuhúsVölundarhús Grandagar›ur Tún Teigar Gagnvegur Fíladelfía Hátún Laugardalshöll Lei›hamrar Vegghamrar Grandi LÆKJARTORG Harpa HLEMMUR MaríubaugurIngunnarskóliPrestastígur -

The Mechanism of Ascus Firing
fungal biology reviews 28 (2014) 70e76 journal homepage: www.elsevier.com/locate/fbr Review The mechanism of ascus firing e Merging biophysical and mycological viewpoints Frances TRAILa,*, Agnese SEMINARAb aDepartment of Plant Biology, Department of Plant, Soil and Microbial Sciences, Michigan State University, East Lansing, MI 48824, USA bCNRS, Laboratoire de physique de la matiere condensee, Parc Valrose, 06108, Nice, France article info abstract Article history: The actively discharging ascus is the unique spore-bearing cell that is responsible to Received 4 November 2012 dispatch spores into the atmosphere. From a physical perspective, this type of ascus is a Received in revised form sophisticated pressure gun that reliably discharges the spores at an extremely high veloc- 19 March 2014 ity, without breaking apart. We identify four essential steps in discharge of spores whose Accepted 17 July 2014 order and timing may vary across species. First, asci that fire are mature, so a cue must be present that prevents discharge of immature spores and signals maturity. Second, pres- Keywords: sure within the ascus serves to propel the spores forward; therefore a mechanism should Apothecia be present to pressurize the ascus. Third, in ostiolate fruiting bodies (e.g. perithecia), the Ascospore ascus extends through an opening to fire spores into the air. The extension process is a Locule relatively unique aspect of the ascus and must be structurally facilitated. Fourth, the ascus Paraphyses must open at its tip for spore release in a controlled rupture. Here we discuss each of these Perithecia aspects in the context of understanding the process of ascus and fruiting body function. -

Iceland: a Laboratory for Non-Indigenous Ascidians
BioInvasions Records (2020) Volume 9, Issue 3: 450–460 CORRECTED PROOF Research Article Iceland: a laboratory for non-indigenous ascidians Alfonso A. Ramos-Esplá1,*, Joana Micael2, Halldór P. Halldórsson3 and Sindri Gíslason2 1Research Marine Centre of Santa Pola (CIMAR), University of Alicante, 03080 Alicante, Spain 2Southwest Iceland Nature Research Centre (SINRC), 245 Suðurnesjabær, Iceland 3University of Iceland, Sudurnes Research Center, 245 Suðurnesjabær, Iceland *Corresponding author E-mail: [email protected] Citation: Ramos-Esplá AA, Micael J, Halldórsson HP, Gíslason S (2020) Abstract Iceland: a laboratory for non-indigenous ascidians. BioInvasions Records 9(3): 450– Non-indigenous species (NIS) represent a serious problem worldwide, where ascidians 460, https://doi.org/10.3391/bir.2020.9.3.01 are one of the most important taxa. However, little has been done to document the non-indigenous ascidians in Iceland, and over the past decade only two species had Received: 30 October 2019 been recorded prior to the present study, Ciona intestinalis in 2007 and Botryllus Accepted: 19 March 2020 schlosseri in 2011. To increase the knowledge of this taxon, extensive sampling Published: 7 May 2020 was carried out in shallow waters around Iceland, during the summer 2018, in ports Handling editor: Noa Shenkar and on ropes of a long-line mussel aquaculture. In total, eleven species were identified, Thematic editor: Stelios Katsanevakis four native and seven NIS, of which Diplosoma listerianum, Ascidiella aspersa, Copyright: © Ramos-Esplá et al. Botrylloides violaceus, Molgula manhattensis and Ciona cf. robusta, are now reported This is an open access article distributed under terms for the first time in Iceland. -

Pálsson, Grétar Már. 2015
Impact on households and critical infrastructures from electricity failure Two case studies and a survey on public preparedness Grétar Már Pálsson Faculty of Civil and Environmental Engineering University of Iceland 2015 Impact on households and critical infrastructures from electricity failure Two case studies and a survey on public preparedness Grétar Már Pálsson 30 ECTS thesis submitted in partial fulfillment of a Magister Scientiarum degree in Civil Engineering Advisors Dr. Björn Karlsson Böðvar Tómasson Faculty Representative Sveinn Júlíus Björnsson Faculty of Civil and Environmental Engineering School of Engineering and Natural Sciences University of Iceland Reykjavik, May 2015 Impact on households and critical infrastructures - Two case studies and a survey on public preparedness. 30 ECTS thesis submitted in partial fulfillment of a Magister Scientiarum degree in civil engineering Copyright © 2015 Grétar Már Pálsson All rights reserved Faculty of Civil and Environmental Engineering School of Engineering and Natural Sciences University of Iceland VR II, Hjarðarhaga 2-6 107, Reykjavik Iceland Telephone: 525 4600 Bibliographic information: Grétar Már Pálsson, 2015, Impact on households and critical infrastructures - Two case studies and a survey on public preparedness, Master’s thesis, Faculty of Civil and Environmental Engineering, University of Iceland, pp. 76. Printing: Háskólaprent, Fálkagata 2, 107 Reykjavík Reykjavik, Iceland, May 2015 Abstract This thesis studies the impact from electricity failure in Iceland on households and critical infrastructures. Households and critical infrastructures electricity dependence is discussed along with a theoretical identification of impacts towards these two subjects from electricity failure. Risk Assessment Plans for Iceland, Norway and Sweden are compared. The main focus of the comparison relates to how the countries focus on electricity, information and communication technologies and the role of the general public in these plans. -

The Xylariaceae As Model Example for a Unified Nomenclature Following
This article was downloaded by: [Helmholtz Zentrum Fuer], [Marc Stadler] On: 13 May 2013, At: 09:12 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Mycology: An International Journal on Fungal Biology Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/tmyc20 The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept Marc Stadler a , Eric Kuhnert a , Derek Peršoh b & Jacques Fournier c a Department Microbial Drugs , Helmholtz-Centre for Infection Research and Technical University of Braunschweig , Inhoffenstrasse 7, 38124 , Braunschweig , Germany b Department of Mycology , University of Bayreuth, Universitätsstrasse 30 , 95440 , Bayreuth , Germany c Las Muros, Rimont , Ariége , 09420 , France To cite this article: Marc Stadler , Eric Kuhnert , Derek Peršoh & Jacques Fournier (2013): The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept, Mycology: An International Journal on Fungal Biology, 4:1, 5-21 To link to this article: http://dx.doi.org/10.1080/21501203.2013.782478 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. -

Initial Assessment of the ISF Iceland Northern Shrimp Fishery (Inshore and Offshore)
FINAL REPORT Initial assessment of the ISF Iceland Northern shrimp fishery (inshore and offshore) Icelandic Sustainable Fisheries Report No.: 2017-032, Rev. 1 Document No.: Date: 2018-10-02 Certificate code: to be determined / xxx Report type: Final Report DNV GL – Business Assurance Report title: Initial assessment of the ISF Iceland Northern shrimp fishery (inshore and offshore) DNV GL Business Assurance Customer: Icelandic Sustainable Fisheries, Grandagarður Norway AS 16, 101 Reykjavík Veritasveien 1 Contact person: Kristinn Hjalmarsson 1322 HØVIK, Norway Date of issue: 2018-10-02 Tel: +47 67 57 99 00 Project No.: PRJC-569364-2017-MSC-NOR http://www.dnvgl.com Organisation unit: Food & Beverage Report No.: 2017-032, Rev.1 Certificate No.: Objective: Assessment of the Iceland Northern Shrimp fishery against MSC Fisheries Standards v2.0. Prepared by: Verified by: Stefan Midteide, Project Manager Sigrun Bekkevold Julian Addison, Expert, Principle 1 Lucia Revenga, Expert Principle 2, Team leader Geir Hønneland, Expert, Principle 3 Copyright © DNV GL 2014. All rights reserved. This publication or parts thereof may not be copied, reproduced or transmitted in any form, or by any means, whether digitally or otherwise without the prior written consent of DNV GL. DNV GL and the Horizon Graphic are trademarks of DNV GL AS. The content of this publication shall be kept confidential by the customer, unless otherwise agreed in writing. Reference to part of this publication which may lead to misinterpretation is prohibited. DNV GL Distribution: Keywords: ☒ Unrestricted distribution (internal and Marine Stewardship Council, Iceland, Northern external) shrimp ☐ Unrestricted distribution within DNV GL ☐ Limited distribution within DNV GL after 3 years ☐ No distribution (confidential) ☐ Secret Rev.