5 Lipoxygenase's Role in Chondrogenesis of the ATDC5 Cell Line Tien My Tran [email protected]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Module 2 : Anatomy – the Skeleton
Module 2 : Anatomy – The Skeleton In this module you will learn: The functions of the skeletal system The types of bones in the human body The effects of exercise on your bones What happens to the bones as we get older When studying to become a fitness instructor or personal trainer, you will learn all about the anatomy of the human body. Studying the skeleton is one of the foundations of your trade, you will need to know how the body is structured, the names of each bone, types of bones, importance of bone and joint health, detail of the spine and different terms of movement. Without stating the obvious, each of your clients has their own skeleton and you must be fully aware of how this works. This is for many reasons; you are a teacher and must be fully aware of how to prevent injuries, avoid unnecessary stress on the bones and, if qualified, help the client prevent or heal bone and joint related conditions or medical problems. 2.1 Understanding the Skeletal System The skeleton is comprised of 206 different bones that provide 5 main functions: Support mechanism for muscle and tissue Protection for organs Movement with bones, muscles, and joints Storing minerals and blood cells Growth Skeletal System 2.2 Bones are Formed by Ossification Some bones (such as the flat bones of your skull) in the body are formed in a similar stage to connective tissue. The process is known as direct or intramembranous ossification. Other bones are made up of cartilaginous matter, this is developed from future bone in the embryo which then dissolves and is replaced with other bone cells. -

Bone Markings / Features on Bones
08/05/2016 Bone Markings : Skeletal System Search Custom Search Like Tweet Home Health News Human Body Biology Chemistry Glossary Textbooks Bone Disorders Ads by Google ► Bone Tissue ► Bone Marrow ► Human Skull Bone ► Bone on Bone Knee Sun 8 May 2016 Bone Markings / Features on Bones Human Body Study Section Bone markings and the features of bones (including the correct words used to describe them) are often required by firstlevel courses in human anatomy and associated health science subjects. It is important to be familiar with the terminology used to Human Body Index refer to bone markings in order to communicate effectively with professionals involved in healthcare, research, forensics, and Health Glossary related disciplines. More about Bones and the Skeletal System: The following terms used to describe bone markings or features on bones are in alphabetical order with short definitions: Human Skeleton Axial and Appendicular Word / Term Meaning / Description Type of Example(s) Skeleton (Bone Marking or bone The Structure and Feature) marking Functions of Bones Types of Bones 1. Angle A corner Feature of Inferior angle (lower) and superior angle (upper) are Bone Markings & Features shape of bone the rounded angles or "corners" of the scapula. on Bones Disorders of the Skeletal 2. Body The main portion of a bone The diaphysis of long bones such as the humerus. System Curvature of the Spine 3. Condyle Rounded bump or large rounded Process The medial condyle of the femur (bone), upperleg. prominence. Such rounded surfaces forms joints Types of Joints usually fit into a fossa on another bone to Specific bones: form a joint. -

The Epiphyseal Plate: Physiology, Anatomy, and Trauma*
3 CE CREDITS CE Article The Epiphyseal Plate: Physiology, Anatomy, and Trauma* ❯❯ Dirsko J. F. von Pfeil, Abstract: This article reviews the development of long bones, the microanatomy and physiology Dr.med.vet, DVM, DACVS, of the growth plate, the closure times and contribution of different growth plates to overall growth, DECVS and the effect of, and prognosis for, traumatic injuries to the growth plate. Details on surgical Veterinary Specialists of Alaska Anchorage, Alaska treatment of growth plate fractures are beyond the scope of this article. ❯❯ Charles E. DeCamp, DVM, MS, DACVS athologic conditions affecting epi foramen. Growth factors and multipotent Michigan State University physeal (growth) plates in imma stem cells support the formation of neo ture animals may result in severe natal bone consisting of a central marrow P 2 orthopedic problems such as limb short cavity surrounded by a thin periosteum. ening, angular limb deformity, or joint The epiphysis is a secondary ossifica incongruity. Understanding growth plate tion center in the hyaline cartilage forming anatomy and physiology enables practic the joint surfaces at the proximal and distal At a Glance ing veterinarians to provide a prognosis ends of the bones. Secondary ossification Bone Formation and assess indications for surgery. Injured centers can appear in the fetus as early Page E1 animals should be closely observed dur as 28 days after conception1 (TABLE 1). Anatomy of the Growth ing the period of rapid growth. Growth of the epiphysis arises from two Plate areas: (1) the vascular reserve zone car Page E2 Bone Formation tilage, which is responsible for growth of Physiology of the Growth Bone is formed by transformation of con the epiphysis toward the joint, and (2) the Plate nective tissue (intramembranous ossifica epiphyseal plate, which is responsible for Page E4 tion) and replacement of a cartilaginous growth in bone length.3 The epiphyseal 1 Growth Plate Closure model (endochondral ossification). -

BONE LANDMARKS Vocabulary to Learn: Bone Structures Attachments
BONE LANDMARKS ✔ Vocabulary to learn: Attachments for Articulation Depressions Bone structures tendons or projections and openings ligaments Articular cartilage Crest Condyle Fissure Compact bone Epicondyle Facet Foramen Diaphysis Line Head Fossa Endosteum Process Ramus Groove Epiphyseal line Spine Meatus Epiphysis Trochanter Nutrient foramen Medullary cavity Tubercle Sinus Neck Tuberosity Sulcus Periosteum Spongy bone Trabeculae ✔ Complete the following statements with the most appropriate term: On the external surface and most of the diaphysis, the superficial structure of the bone is dense or ________________________ bone, whereas internally and deep in the epiphyses, the bone is open or ________________________ bone and consists of ________________________, which are a fine meshwork of "flying buttresses" to give the bone strength. The tubular shaft of the bone is called the ________________________ which in some cases contains a medullary cavity. The extremity of the bone is called the ________________________ and it is distally covered by a thin layer of ________________________. Bone is covered by a dense connective tissue called ________________________. A narrowed portion of a bone at the base of the head of the bone is the ________________________. A moderately raised ridge along a bone for attaching muscles is a ________________________ or line. A sharp, slender pointed projection (like the point of a pencil) is a ________________________ and is the site of attachment for muscles or ligaments. Any bony projection or bump can be generically called a _______________________. A shallow depression in a bone is called a ________________________. Openings in a bone, for passage of a nerve or vessel, that are round or oval are referred to as ________________________ , ________________________ if they are narrow or slit-like, and a ________________________ if they are canal-like. -

Compact Bone Spongy Bone
Spongy bone Compact bone © 2018 Pearson Education, Inc. 1 (b) Flat bone (sternum) (a) Long bone (humerus) (d) Irregular bone (vertebra), right lateral view (c) Short bone (talus) © 2018 Pearson Education, Inc. 2 Articular cartilage Proximal epiphysis Spongy bone Epiphyseal line Periosteum Compact bone Medullary cavity (lined by endosteum) Diaphysis Distal epiphysis (a) © 2018 Pearson Education, Inc. 3 Trabeculae of spongy bone Osteon (Haversian Perforating system) (Volkmann’s) canal Blood vessel continues into medullary cavity containing marrow Blood vessel Lamellae Compact bone Central (Haversian) canal Perforating (Sharpey’s) fibers Periosteum Periosteal blood vessel (a) © 2018 Pearson Education, Inc. 4 Lamella Osteocyte Canaliculus Lacuna Central Bone matrix (Haversian) canal (b) © 2018 Pearson Education, Inc. 5 Osteon Interstitial lamellae Lacuna Central (Haversian) canal (c) © 2018 Pearson Education, Inc. 6 Articular cartilage Hyaline Spongy cartilage bone New center of bone growth New bone Epiphyseal forming plate cartilage Growth Medullary in bone cavity width Bone starting Invading to replace Growth blood cartilage in bone vessels length New bone Bone collar forming Hyaline Epiphyseal cartilage plate cartilage model In an embryo In a fetus In a child © 2018 Pearson Education, Inc. 7 Bone growth Bone grows in length because: Articular cartilage 1 Cartilage grows here. Epiphyseal plate 2 Cartilage is replaced by bone here. 3 Cartilage grows here. © 2018 Pearson Education, Inc. 8 Bone remodeling Growing shaft is remodeled as: Articular cartilage Epiphyseal plate 1 Bone is resorbed by osteoclasts here. 2 Bone is added (appositional growth) by osteoblasts here. 3 Bone is resorbed by osteoclasts here. © 2018 Pearson Education, Inc. 9 Hematoma External Bony callus callus of spongy bone New Internal blood callus vessels Healed (fibrous fracture tissue and Spongy cartilage) bone trabecula 1 Hematoma 2 Fibrocartilage 3 Bony callus 4 Bone remodeling forms. -

The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: a Review
cells Review The Potential of FGF-2 in Craniofacial Bone Tissue Engineering: A Review Anita Novais 1,2, Eirini Chatzopoulou 1,2,3, Catherine Chaussain 1,2 and Caroline Gorin 1,2,* 1 Pathologies, Imagerie et Biothérapies Orofaciales, Université de Paris, URP2496, 1 rue Maurice Arnoux, 92120 Montrouge, France; [email protected] (A.N.); [email protected] (E.C.); [email protected] (C.C.) 2 AP-HP Département d’Odontologie, Services d’odontologie, GH Pitié Salpêtrière, Henri Mondor, Paris Nord, Hôpital Rothschild, Paris, France 3 Département de Parodontologie, Université de Paris, UFR Odontologie-Garancière, 75006 Paris, France * Correspondence: [email protected]; Tel./Fax: +33-(0)1-5807-6724 Abstract: Bone is a hard-vascularized tissue, which renews itself continuously to adapt to the mechanical and metabolic demands of the body. The craniofacial area is prone to trauma and pathologies that often result in large bone damage, these leading to both aesthetic and functional complications for patients. The “gold standard” for treating these large defects is autologous bone grafting, which has some drawbacks including the requirement for a second surgical site with quantity of bone limitations, pain and other surgical complications. Indeed, tissue engineering combining a biomaterial with the appropriate cells and molecules of interest would allow a new therapeutic approach to treat large bone defects while avoiding complications associated with a second surgical site. This review first outlines the current knowledge of bone remodeling and the different signaling pathways involved seeking to improve our understanding of the roles of each to be able to stimulate or inhibit them. -

Aandp1ch07lecture.Pdf
Chapter 07 Lecture Outline See separate PowerPoint slides for all figures and tables pre- inserted into PowerPoint without notes. Copyright © McGraw-Hill Education. Permission required for reproduction or display. 1 Introduction • In this chapter we will cover: – Bone tissue composition – How bone functions, develops, and grows – How bone metabolism is regulated and some of its disorders 7-2 Introduction • Bones and teeth are the most durable remains of a once-living body • Living skeleton is made of dynamic tissues, full of cells, permeated with nerves and blood vessels • Continually remodels itself and interacts with other organ systems of the body • Osteology is the study of bone 7-3 Tissues and Organs of the Skeletal System • Expected Learning Outcomes – Name the tissues and organs that compose the skeletal system. – State several functions of the skeletal system. – Distinguish between bones as a tissue and as an organ. – Describe the four types of bones classified by shape. – Describe the general features of a long bone and a flat bone. 7-4 Tissues and Organs of the Skeletal System • Skeletal system—composed of bones, cartilages, and ligaments – Cartilage—forerunner of most bones • Covers many joint surfaces of mature bone – Ligaments—hold bones together at joints – Tendons—attach muscle to bone 7-5 Functions of the Skeleton • Support—limb bones and vertebrae support body; jaw bones support teeth; some bones support viscera • Protection—of brain, spinal cord, heart, lungs, and more • Movement—limb movements, breathing, and other -
Skeletal System and Bones References: • Eizenberg, Briggs, Et Al
Lecture 19: Skeletal System and Bones References: • Eizenberg, Briggs, et al. 2008, ‘General Anatomy: Principles and Applications’. Ch 4, pp 25-35 • Anatomedia CD ROM: General Anatomy: Systems Frames 1-9 • Drake et al. 2010: Gray’s Anatomy for Students: Ch 1, pp 14-20 Skeletal Framework of Body: Subdivided into: Axial Skeleton: - Forms the axis of the body - Made of: Skull, vertebral Column, ribs, sternum Appendicular Skeleton: - The “appendages” that attach to the axial - Includes: limbs and limb girdles (what attaches the limbs to the axial skeleton) - Eg. pelvic girdles—made up the pelvis (part of the appendicular skeleton) - Eg. shoulder girdle—made up of scapula and clavicle Skeletal system: - Made up of bones and cartilage Function of skeletal system: - Supports the body and muscles - Protects and encloses visceral organs (organ surrounded by bones) eg. brain, heart, lungs - Helps movement – because muscles attached to bones - Blood formation in bone marrow (RBC) - Stores minerals & salts: o Eg. calcium, phosphorous o Provides electrolyte balance and releases them when body is needed - Removes foreign and toxic heavy metals o The minerals and salt in bones can attract heavy metals o Immune can recognize toxic metals and remove it Types of Bones 206 Bones in adult body—children have greater number of bones that adult because individual bones have not fused yet Bones of appendicular system: Long: Flat bones: - Arm, leg, digits bones - Enclose structures - Long hollow tubular structure - Cranial bones—enclose brains Short bones: - Sternum, mandible - Carpal bones—bones in wrist Irregular shaped bones - Vertebrae - Tarsal bones—bones in ankles - Bones of the face - They are cuboidal in shape (not metacarpals/tarsals though—long bone) Other bones that are a mix of all of these: - Pneumatic bones: o air filled so these bones are very light o eg. -

Skeletal System Day 1 Review
Skeletal System Day 1 Review Directions: Answer the review question based on what was covered in the notes. The 4 review worksheets will be !your “study guide(s)” for your unit test. 1. Ligaments attach ___________ to ____________ where tendons attach ____________ to ____________. 2. What are the 2 divisions of the skeletal system? ______________________ & _______________________ 3. List 5 functions of the skeletal system. - - - - - 4. How many bones are in the adult skeleton? ________________ !5. What is the difference between compact bone and spongy bone? ! ! ! 6. What are the 4 shapes of bones? ______________________ ______________________ ______________________ _______________________ 7. What type of bone are “long bones” made of? _______________________ 8. What type of bone are “short bones” made of? _______________________ 9. What type(s) of bone are “flat bones” made of? ___________________________________________________ !10. What is the diaphysis and epiphysis made up of (type of bone)? 11. Define and Label the structures of a Long Bone: ! d. ! ! ! 1. ! ! ! !a. e. ! ! ! ! f. ! ! g. ! 2. ! ! ! ! h. ! 3. ! ! !!! !b. ! 4. ! ! 5.! 6. x !c. 12. What is the main function of articular cartilage? Where is the location of this form of cartilage? What is it !made of? ! !13. What does yellow bone marrow contain? Red bone marrow? ! !14. What is a “process” or “projection” of a bone? “depression” or “cavity”? ! 15. In an embryo (in womb) what is the skeleton made up of? __________________________________ 16. What is the technical term for “growth plate” _____________________________________________ 17. What is the process of transforming cartilage into bone? (as a baby grows) ! 18. Define the following terms: -Osteocytes: -Osteoblasts: -Osteoclasts: 19. -

Musculoskeletal System
4 Musculoskeletal System Learning Objectives Upon completion of this chapter, you will be able to • Identify and define the combining forms, prefixes, and suffixes introduced in this chapter. • Correctly spell and pronounce medical terms and major anatomical structures relating to the musculoskeletal system. • Locate and describe the major organs of the musculoskeletal system and their functions. • Correctly place bones in either the axial or the appendicular skeleton. • List and describe the components of a long bone. • Identify bony projections and depressions. • Identify the parts of a synovial joint. • Describe the characteristics of the three types of muscle tissue. • Use movement terminology correctly. • Identify and define musculoskeletal system anatomical terms. • Identify and define selected musculoskeletal system pathology terms. • Identify and define selected musculoskeletal system diagnostic procedures. • Identify and define selected musculoskeletal system therapeutic procedures. • Identify and define selected medications relating to the musculoskeletal system. • Define selected abbreviations associated with the musculoskeletal system. 83 M04_FREM0254_06_SE_C04.indd 83 18/12/14 10:12 pm Section I: Skeletal System at a Glance Function The skeletal system consists of 206 bones that make up the internal framework of the body, called the skeleton. The skeleton supports the body, protects internal organs, serves as a point of attachment for skeletal muscles for body movement, produces blood cells, and stores minerals. Organs Here -
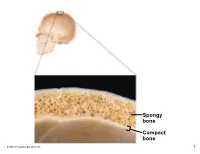
Compact Bone Spongy Bone
Spongy bone Compact bone © 2018 Pearson Education, Inc. 1 (b) Flat bone (sternum) (a) Long bone (humerus) (d) Irregular bone (vertebra), right lateral view (c) Short bone (talus) © 2018 Pearson Education, Inc. 2 Articular cartilage Proximal epiphysis Spongy bone Epiphyseal line Periosteum Compact bone Medullary cavity (lined by endosteum) Diaphysis Distal epiphysis (a) © 2018 Pearson Education, Inc. 3 Trabeculae of spongy bone Osteon (Haversian Perforating system) (Volkmann’s) canal Blood vessel continues into medullary cavity containing marrow Blood vessel Lamellae Compact bone Central (Haversian) canal Perforating (Sharpey’s) fibers Periosteum Periosteal blood vessel (a) © 2018 Pearson Education, Inc. 4 Lamella Osteocyte Canaliculus Lacuna Central Bone matrix (Haversian) canal (b) © 2018 Pearson Education, Inc. 5 Osteon Interstitial lamellae Lacuna Central (Haversian) canal (c) © 2018 Pearson Education, Inc. 6 Articular cartilage Hyaline Spongy cartilage bone New center of bone growth New bone Epiphyseal forming plate cartilage Growth Medullary in bone cavity width Bone starting Invading to replace Growth blood cartilage in bone vessels length New bone Bone collar forming Hyaline Epiphyseal cartilage plate cartilage model In an embryo In a fetus In a child © 2018 Pearson Education, Inc. 7 Bone growth Bone grows in length because: Articular cartilage 1 Cartilage grows here. Epiphyseal plate 2 Cartilage is replaced by bone here. 3 Cartilage grows here. © 2018 Pearson Education, Inc. 8 Bone remodeling Growing shaft is remodeled as: Articular cartilage Epiphyseal plate 1 Bone is resorbed by osteoclasts here. 2 Bone is added (appositional growth) by osteoblasts here. 3 Bone is resorbed by osteoclasts here. © 2018 Pearson Education, Inc. 9 Hematoma External Bony callus callus of spongy bone New Internal blood callus vessels Healed (fibrous fracture tissue and Spongy cartilage) bone trabecula 1 Hematoma 2 Fibrocartilage 3 Bony callus 4 Bone remodeling forms. -

Chapter 6 Bone Tissue and the Skeletal System an Introduction to the Skeletal System
Chapter 6 Bone Tissue and the Skeletal System An Introduction to the Skeletal System . Skeletal system includes . Bones of the skeleton . Cartilages, ligaments, and connective tissues Introduction video 10 min CrashCourse The Axial Skeleton 6-1 Functions of the Skeletal System . Support . Storage of minerals (calcium) . Storage of lipids (yellow marrow) . Blood cell production (red marrow) . Protection . Leverage (force of motion) 6-2 Classification of Bones . Bones are classified by . Shape . Internal tissue organization . Bone markings (surface features; marks) Classification of Bones Classification of Bones by Shape. Classification of Bones . Bone Shapes . Long bones . Are long and thin . Are found in arms, legs, hands, feet, fingers, and toes . Flat bones . Are thin with parallel surfaces . Are found in the skull, sternum, ribs, and scapulae . Sutural bones . Are small, irregular bones . Are found between the flat bones of the skull Classification of Bones . Bone Shapes . Irregular bones . Have complex shapes . Examples: spinal vertebrae, pelvic bones . Short bones . Are small and thick . Examples: ankle and wrist bones . Sesamoid bones . Are small and flat . Develop inside tendons near joints of knees, hands, and feet Classification of Bones Copyright © 2009 Pearson Education, Inc., publishing as Pearson Benjamin Cummings 6-3 Bone Structure . Structure of a Long Bone- sections called . Diaphysis . The shaft . A heavy wall of compact bone, or dense bone . A central space called medullary (marrow) cavity . Epiphysis . Wide part at each end . Articulation with other bones . Mostly spongy (cancellous) bone . Covered with compact bone (cortex) . Metaphysis . Where diaphysis and epiphysis meet Gross Anatomy of a Bone Outside- Compact dense bone- Osteons Framework Inside- Spongy bone –also called cancellous with trabeculae( bridgework) Cavities filled with bone marrow Middle cavity- Medullary Gross Anatomy .