A Collagen/Laminin Receptor on Others MARIANO J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Collagen and Elastin Fibres
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.49 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 49-58 Collagen and elastin fibres A. J. BAILEY From the Agricultural Research Council, Meat Research Institute, Langford, Bristol Although an understanding of the intracellular native collagen was generated from type I pro- biosynthesis of both collagen and elastin is of collagen. Whether this means that the two pro- considerable importance it is the subsequent extra- collagens are converted by different enzyme systems cellular changes involving fibrogenesis and cross- and the type III enzyme was deficient in these linking that ensure that these proteins ultimately fibroblast cultures, or that the processing of pro become the major supporting tissues of the body. type III is extremely slow, is not known. The latter This paper summarises the formation and stability proposal is consistent with the higher proportion of collagen and elastin fibres. of soluble pro type III extractable from tissue (Lenaers and Lapiere, 1975; Timpl et al., 1975). Collagen Basement membrane collagens, on the other hand, do not form fibres and this property may be The non-helical regions at the ends of the triple due to the retention of the non-helical extension helix of procollagen probably provide a number of peptides (Kefalides, 1973). In-vivo biosynthetic different intracellular functions-that is, initiating studies showing the absence of any extension peptide rapid formation of the triple helix; inhibiting intra- removal support this (Minor et al., 1976), but other cellular fibrillogenesis; and facilitating transmem- workers have reported that there is some cleavage brane movement. -

Evaluation of Elastin/Collagen Content in Human Dermis In-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging
Cosmetics 2014, 1, 211-221; doi:10.3390/cosmetics1030211 OPEN ACCESS cosmetics ISSN 2079-9284 www.mdpi.com/journal/cosmetics Article Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging Jean-Christophe Pittet 1,*, Olga Freis 2,†, Marie-Danielle Vazquez-Duchêne 2,†, Gilles Périé 2,† and Gilles Pauly 2,† 1 Orion Concept, 100 Rue de Suède, 37100 Tours, France 2 BASF Beauty Care Solutions France SAS, 3 Rue de Seichamps, CS 71040 Pulnoy, 54272 Essey-lès-Nancy Cedex, France; E-Mails: [email protected] (O.F.); [email protected] (M.-D.V.-D.); [email protected] (G.Pé.); [email protected] (G.Pa.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-247-052-316; Fax: +33-610-786-695. Received: 14 March 2014; in revised form: 31 July 2014 / Accepted: 1 August 2014 / Published: 20 August 2014 Abstract: The aim of this study was to evaluate the influence of the depth of the dermis on the measured collagen and elastin levels and to establish the correlation between the amount of these two extracellular matrix (ECM) components and age. Multiphoton Microscopy (MPM) that measures the autofluorescence (AF) and second harmonic generation (SHG) was used to quantify the levels of elastin and collagen and to determine the SAAID (SHG-to-AF Aging Index of Dermis) at two different skin depths. A 50 MHz ultrasound scanner was used for the calculation of the Sub Epidermal Non Echogenic Band (SENEB). -

Blood Vitronectin Is a Major Activator of LIF and IL-6 in the Brain Through Integrin–FAK and Upar Signaling Matthew P
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs202580. doi:10.1242/jcs.202580 RESEARCH ARTICLE Blood vitronectin is a major activator of LIF and IL-6 in the brain through integrin–FAK and uPAR signaling Matthew P. Keasey1, Cuihong Jia1, Lylyan F. Pimentel1,2, Richard R. Sante1, Chiharu Lovins1 and Theo Hagg1,* ABSTRACT Microglia and astrocytes express the VTN receptors αvβ3 and α β We defined how blood-derived vitronectin (VTN) rapidly and potently v 5 integrin (Herrera-Molina et al., 2012; Kang et al., 2008; activates leukemia inhibitory factor (LIF) and pro-inflammatory Milner, 2009; Welser-Alves et al., 2011). Microglia and astrocytes, interleukin 6 (IL-6) in vitro and after vascular injury in the brain. as well as endothelial cells, are major producers of pro- α in vitro Treatment with VTN (but not fibrinogen, fibronectin, laminin-111 or inflammatory cytokines, such as IL-6 and TNF , and collagen-I) substantially increased LIF and IL-6 within 4 h in after traumatic or ischemic injury to the brain (Banner et al., 1997; C6-astroglioma cells, while VTN−/− mouse plasma was less effective Erta et al., 2012; Lau and Yu, 2001) or upon self-induction by IL-6 than that from wild-type mice. LIF and IL-6 were induced by (Van Wagoner and Benveniste, 1999). IL-6 is a major regulator of a intracerebral injection of recombinant human (rh)VTN in mice, but variety of inflammatory disorders and a target for therapies (Hunter induction seen upon intracerebral hemorrhage was less in VTN−/− and Jones, 2015). -

With Caviar, Keratin & Collagen
WITH CAVIAR, KERATIN & COLLAGEN Professional treatments for colour, bleaching, care and maintenance with CAVIAR, KERATIN and COLLAGEN Professional styling products with CAVIAR, KERATIN and COLLAGEN Technical professional products with CAVIAR, KERATIN and COLLAGEN 2 Professional products by Very high technology professional products to colour, treat and protect hair from the continuous chemical and environmental stress caused on a daily basis. Formulas based on: Caviar Keratin Collagen 3 WITH CAVIAR, KERATIN & COLLAGEN Colouring permanentCream professional ammonia PPD • Respects the hair structure thanks to an exposure free free time of 12 minutes • Non-progressive • Ammonia free • Paraphenylenediamine free • Unleashes all the EFFICANCY of its active principles and maximum COLOURING POWER Mix 1 : 1 4 WITH CAVIAR, KERATIN & COLLAGEN 1. Respect for scalp and hair thanks to a shorter processing time 2. Maximum grey hair coverage 3. Lightens up to 4 tones 4. Ammonia free and Paraphenylenediamine free 5. Safe application even on customers with a sensitive scalp 6. Extreme colour brilliancy and uniformity 7. Very easy and practical to use 8. Long lasting reflections 9. High colour fastness 10. Great protection action 11. Effective restructuring action benefits 12. Maximum conditioning 5 WITH CAVIAR, KERATIN & COLLAGEN Benefits 1 2 3 Respect for scalp and hair thanks to a shorter processing Lightens up to Maximum grey hair 4 tones time coverage 6 WITH CAVIAR, KERATIN & COLLAGEN has been developed according Cosmetic colour pDT BASE Be Colour 12 Minute Dpe DIAMINOPHENOXYETHANOL to the rules of the “molar stoichiometry,” a technique creamy gel with RESORCINOL m-AMINOPHENOL of colouring clear and uncompromising. It is based on CAVIAR, a mathematical principle according to which the molar concentration of the dye base is equal to the molar KERATIN and concentration of the sum of the other colouring couplers. -
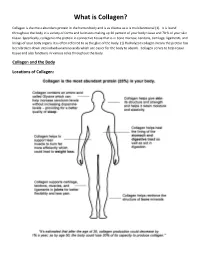
What Is Collagen?
What is Collagen? Collagen is the most abundant protein in the human body and is as diverse as it is multifunctional (1). It is found throughout the body in a variety of forms and functions making up 30 percent of your body tissue and 70 % of your skin tissue. Specifically, collagen is the protein in connective tissue that is in bone marrow, tendons, cartilage, ligaments, and linings of your body organs. It is often referred to as the glue of the body. (1) Hydrolyzed collagen means the protein has been broken down into individual amino acids which are easier for the body to absorb. Collagen serves to help repair tissue and also functions in various roles throughout the body. Collagen and the Body Locations of Collagen: What Does Collagen Do? Collagen has numerous structural properties but also plays a vital role in the repair of almost all the body’s tissues. Some diseases are directly linked to lacking this essential protein. Depending on which part of the body it is located, collagen serves different purposes. In skin: Found in the inner layer, this connective tissue gives the skin its structure and strength and also functions in the replacement of dead skin cells. A lack of collagen in the skin can contribute to a decrease in skin health leading to stretch marks, dark spots, and infections as well as affecting the skin’s ability to maintain moisture. In internal organs and blood vessels: In the lining of your organs like in the stomach, kidneys, blood vessels and spleen, collagen functions as a protective covering and a fibrous barrier. -

Empowering Collagen Targeting for Diagnosis and Treatment of Human Conditions
EmpoweringEmpowering collagen collagen targetingtargeting for for diagnosis prognosis of fibrotic and treatment of Manka,SW. 2012 conditions.human conditions. 1 3Helix as a platform diagnostic company 2020 2022 2030 Innovative research reagent for Clinic histopathology providing Platform fibrotic prognostic detection of collagen damage best in class prognostic ability in within multi-disease states liver fibrosis (NAFLD, NASH) Strengthening IP portfolio with market Clinical histopathology AND Non-Invasive approaches serum testing and medical disrupting products while subsequently Analytic specific reagent to allow for fast imaging developing strong partnerships with world market access leading companies for commercialization Targeting impactful markets of IPF, kidney Focused on paired biopsy research and fibrosis, AMD, Keloids and cardiac fibrosis collaboration with clinical laboratories. in addition to fibrotic liver diseases. 2 Liver Fibrosis market is GROWING Fatty Liver Fibrotic Liver Healthy Liver Cirrhosis NAFLD NASH USA Epidemiology 328 Million 83 Million 16 Million 1.5 Million (2015) Predicted USA Epidemiology 360 Million 101 Million 27 Million 3.4 Million (2030) Estes,C. 2018 3 NASH Therapeutics are finishing clinical trials and are coming to market. • VK2809 Phase II • OCALIVA (OCA) • NDA Filed • $78,000/year current cost • $20,000 predicted • Resmetirom Phase III • 23% respond to treatment 16 Million NASH patients in the USA * $20,000 • $320 Billion Annual Cost for treatable market Aramchol Phase III/IV 4 Stratification of patient population is needed to reduce unnecessary therapeutic intervention. • Progression of NAFL and 100% NASH is variable patient to NAFLD patient. 33% • Prediction of the progression can modify the Fibrosis Progression 20% disease intervention and treatment. Rapid Fibrosis • No product on the market Progression (stage 0 to stage today is equipped for 3/4 over 5.9 years) prognosis of liver fibrosis Singh, S. -

A Comparison of in Vivo Gene Delivery Methods for Antisense Therapy in Ligament Healing
Gene Therapy (1998) 5, 1455–1461 1998 Stockton Press All rights reserved 0969-7128/98 $12.00 http://www.stockton-press.co.uk/gt A comparison of in vivo gene delivery methods for antisense therapy in ligament healing N Nakamura1, SA Timmermann1, DA Hart1, Y Kaneda2, NG Shrive1, K Shino3, T Ochi3 and CB Frank1 1McCaig Centre for Joint Injury and Arthritis Research, University of Calgary, Calgary, Alberta, Canada; 2Institute for Molecular and Cellular Biology, Osaka University; and 3Department of Orthopaedic Surgery, Osaka University Medical School, Osaka, Japan To determine the most efficient in vivo delivery method of at 7 days after transfection. We then introduced antisense oligonucleotides for antisense therapy in ligament healing, ODN for the rabbit proteoglycan, decorin, into ligament fluorescence-labelled phosphorothioate oligodeoxynuleo- scars with this delivery method and confirmed a significant tides (ODN) were introduced into 12 rabbit ligament scars inhibition of decorin mRNA expression in antisense-treated 2 weeks after injury using haemagglutinating virus of Japan scar tissues in vivo both at 2 days (42.3 ± 14.7% of sense (Sendai virus; HVJ)-conjugated liposomes. We compared control ± s.d.; P Ͻ 0.0025) and 3 weeks (60.5 ± 28.2% of the efficiency of cellular uptake of fluorescence as a per- sense control ± s.d.; P Ͻ 0.024) after treatment, compared centage of all cells in each scar using three delivery pro- with sense ODN-treated scars. Decorin was significantly cedures: (1) direct free-hand injection into the ligament suppressed also at protein level in antisense-treated scars scar using a conventional syringe; (2) systematic direct at 4 weeks (66.6 ± 35.7% of sense control ± s.d.; scar injection using a repeating 10 l dispenser and a P Ͻ 0.045) after treatment. -

Collagens—Structure, Function, and Biosynthesis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of East Anglia digital repository Advanced Drug Delivery Reviews 55 (2003) 1531–1546 www.elsevier.com/locate/addr Collagens—structure, function, and biosynthesis K. Gelsea,E.Po¨schlb, T. Aignera,* a Cartilage Research, Department of Pathology, University of Erlangen-Nu¨rnberg, Krankenhausstr. 8-10, D-91054 Erlangen, Germany b Department of Experimental Medicine I, University of Erlangen-Nu¨rnberg, 91054 Erlangen, Germany Received 20 January 2003; accepted 26 August 2003 Abstract The extracellular matrix represents a complex alloy of variable members of diverse protein families defining structural integrity and various physiological functions. The most abundant family is the collagens with more than 20 different collagen types identified so far. Collagens are centrally involved in the formation of fibrillar and microfibrillar networks of the extracellular matrix, basement membranes as well as other structures of the extracellular matrix. This review focuses on the distribution and function of various collagen types in different tissues. It introduces their basic structural subunits and points out major steps in the biosynthesis and supramolecular processing of fibrillar collagens as prototypical members of this protein family. A final outlook indicates the importance of different collagen types not only for the understanding of collagen-related diseases, but also as a basis for the therapeutical use of members of this protein family discussed in other chapters of this issue. D 2003 Elsevier B.V. All rights reserved. Keywords: Collagen; Extracellular matrix; Fibrillogenesis; Connective tissue Contents 1. Collagens—general introduction ............................................. 1532 2. Collagens—the basic structural module......................................... -

Sources of Collagen for Biomaterials in Skin Wound Healing
bioengineering Review Sources of Collagen for Biomaterials in Skin Wound Healing Evan Davison-Kotler 1,2, William S. Marshall 1 and Elena García-Gareta 2,* 1 Biology Department, St. Francis Xavier University, Antigonish, NS B2G 2W5, Canada 2 Regenerative Biomaterials Group, The RAFT Institute, Mount Vernon Hospital, Northwood HA6 2RN, UK * Correspondence: [email protected]; Tel.: +44-(0)-1923844350 Received: 23 May 2019; Accepted: 26 June 2019; Published: 30 June 2019 Abstract: Collagen is the most frequently used protein in the fields of biomaterials and regenerative medicine. Within the skin, collagen type I and III are the most abundant, while collagen type VII is associated with pathologies of the dermal–epidermal junction. The focus of this review is mainly collagens I and III, with a brief overview of collagen VII. Currently, the majority of collagen is extracted from animal sources; however, animal-derived collagen has a number of shortcomings, including immunogenicity, batch-to-batch variation, and pathogenic contamination. Recombinant collagen is a potential solution to the aforementioned issues, although production of correctly post-translationally modified recombinant human collagen has not yet been performed at industrial scale. This review provides an overview of current collagen sources, associated shortcomings, and potential resolutions. Recombinant expression systems are discussed, as well as the issues associated with each method of expression. Keywords: collagen; collagen sources; recombinant collagen; biomaterials; regenerative medicine; tissue engineering; wound healing; skin 1. Introduction 1.1. The Molecular Structure of Collagen The 28 different collagen types of the collagen superfamily are further divided into eight subfamilies, with the majority of the collagen types belonging to the fibril-forming or fibrillar subfamily [1,2]. -

Trends in Collagen Jorge Sarasqueta - Director of Latam
Trends in Collagen Jorge Sarasqueta - Director of Latam May 2017 About Innova Market Insights • Leading global market research company focused on trends and innovation in F&B and ingredients. • We serve our clients with our Innova Database, which the is largest F&B new product launch database: • Based on a network of F&B professionals • Tracking new products in more than 75 countries • Adding more than 200,000 new products per year 2 2 Trends in Collagen TOPICS FOR TODAY 1. Overview of collagen NPD 2. Application of collagen 3. What’s next? 4. Overview of gelatin NPD 5. Application of gelatin 3 3 Overview of collagen NPD Collagen is on trend; steady growth over past 10 years • Launches containing collagen have been rising for past ten years: • Most of growth is attributed to supplements and sport nutrition. Index number of new product launches of food & beverages* tracked with collagen (Global, 2007=100) CAGR 33% 1400 2007 -2016 1200 1000 800 600 400 Index number of new product launches product new of number Index 200 0 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 5 5 *: food & beverages exclude meat, fish & eggs, pet food, oral care and clinical nutrition. Latin America and Asia are catching up the developed market for collagen use • Latin American and Asian markets show the fastest growth in terms of collagen applications, featuring CAGR of 31% and 28% respectively over the past five years. +10%* Europe +18%* +28%* Asia North America +31%* Latin America 6 6 *: CAGR of the food & beverage (exclude meat, fish & eggs, pet food, oral care and clinical nutrition) new product launches tracked with collagen from 2012 to 2016 Functional benefits drive application in supplements • Supplements and sports nutrition lead collagen applications over past 5 years. -

Collagen of the Extracellular Matrix. Functional and Structural Studies
Collagen of the extracellular matrix. Functional and structural studies. Olsson, Olof 2017 Document Version: Publisher's PDF, also known as Version of record Link to publication Citation for published version (APA): Olsson, O. (2017). Collagen of the extracellular matrix. Functional and structural studies. Lund University: Faculty of Medicine. Total number of authors: 1 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 PER OL O F OLSS O N Collagen the of extracellular matrix in carcinoma Collagen of the extracellular matrix in carcinoma Functional and structural studies PER OLOF OLSSON DEPARTMENT OF LABORATORY MEDICINE | LUND UNIVERSITY 2017 Functional and structural studies structural and Functional Lund University, Faculty of Medicine 193907 Doctoral Dissertation Series 2017:9 ISBN 978-91-7619-390-7 ISSN 1652-8220 789176 9 9 Collagen of the extracellular matrix in carcinoma Functional and structural studies 1 2 Collagen of the extracellular matrix in carcinoma Functional and structural studies Per Olof Olsson DOCTORAL DISSERTATION By due permission of the Facultry of Medicine, Lund University, Sweden. -

Chapter 8: Fibrous Proteins
ChapterChapter 8:8: FibrousFibrous ProteinsProteins VoetVoet && Voet:Voet: PagesPages 231-240231-240 Lecture 11 Biochemistry 3100 Slide 1 FibrousFibrous ProteinsProteins Fibrous proteins are highly elongated polypeptides composed of a single secondary structure element Primary component of skin, tendon, bone, connective tissues, etc Function as structural material that have protective, connective or supportive roles Simplicity of structure makes relation between structure and function relatively obvious Rarely crystallize (also difficult for NMR) so structural information a variety of indirect techniques Lecture 11 Biochemistry 3100 Slide 2 KeratinsKeratins Mechanically durable and unreactive protein of vertebrates up to 85% of protein in horns, hair, nails & feathers a-keratins occur in mammals; b-keratins occur in birds and reptiles > 30 keratin genes expressed in mammals a-keratins are classified as relatively acidic (Type I) or basic (Type II) Keratins have complex quaternary structures (a) keratins are dimers composed of a Type I and Type II subunit (b) many dimers associate to form protofilaments (c) protofilaments dimerize to form protofibrils (d) protofibrils form tetramers called microfibrils (e) microfibrils associate into macrofibrils Lecture 11 Biochemistry 3100 Slide 3 KeratinKeratin QuaternaryQuaternary StructureStructure Lecture 11 Biochemistry 3100 Slide 4 aa-Keratin-Keratin StructureStructure X-ray scattering and diffraction studies indicate a- keratin has a helical structure with a 5.1 Å pitch (distance per turn of helix)