Hydrolyzed Collagen—Sources and Applications
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

POLYMER STRUCTURE and CHARACTERIZATION Professor
POLYMER STRUCTURE AND CHARACTERIZATION Professor John A. Nairn Fall 2007 TABLE OF CONTENTS 1 INTRODUCTION 1 1.1 Definitions of Terms . 2 1.2 Course Goals . 5 2 POLYMER MOLECULAR WEIGHT 7 2.1 Introduction . 7 2.2 Number Average Molecular Weight . 9 2.3 Weight Average Molecular Weight . 10 2.4 Other Average Molecular Weights . 10 2.5 A Distribution of Molecular Weights . 11 2.6 Most Probable Molecular Weight Distribution . 12 3 MOLECULAR CONFORMATIONS 21 3.1 Introduction . 21 3.2 Nomenclature . 23 3.3 Property Calculation . 25 3.4 Freely-Jointed Chain . 27 3.4.1 Freely-Jointed Chain Analysis . 28 3.4.2 Comment on Freely-Jointed Chain . 34 3.5 Equivalent Freely Jointed Chain . 37 3.6 Vector Analysis of Polymer Conformations . 38 3.7 Freely-Rotating Chain . 41 3.8 Hindered Rotating Chain . 43 3.9 More Realistic Analysis . 45 3.10 Theta (Θ) Temperature . 47 3.11 Rotational Isomeric State Model . 48 4 RUBBER ELASTICITY 57 4.1 Introduction . 57 4.2 Historical Observations . 57 4.3 Thermodynamics . 60 4.4 Mechanical Properties . 62 4.5 Making Elastomers . 68 4.5.1 Diene Elastomers . 68 0 4.5.2 Nondiene Elastomers . 69 4.5.3 Thermoplastic Elastomers . 70 5 AMORPHOUS POLYMERS 73 5.1 Introduction . 73 5.2 The Glass Transition . 73 5.3 Free Volume Theory . 73 5.4 Physical Aging . 73 6 SEMICRYSTALLINE POLYMERS 75 6.1 Introduction . 75 6.2 Degree of Crystallization . 75 6.3 Structures . 75 Chapter 1 INTRODUCTION The topic of polymer structure and characterization covers molecular structure of polymer molecules, the arrangement of polymer molecules within a bulk polymer material, and techniques used to give information about structure or properties of polymers. -

COLLAGEN DRINK Look Youthful, Fresh and Healthy
Executive Summary COLLAGEN DRINK Look youthful, fresh and healthy Collagen is a natural protein component, the main building block for cells, tissues and organs. The combination of collagen and hyaluronic acid gives healthy joints and moisturized skin. It is odorless and easy to dissolve in liquid so that you can put it in your drinks such as coffee, tea and soup. It is beneficial for healthy joints and moisturized skin. Collagen beauty drinks keep the skin smooth and more youthful. 1 WWW.NIZONA.CO Drink COLLAGEN DRINK Benefits > It is an anti-aging & beauty supplement drink Explanation of Ingredients > Hydrolyzed collagen gelatin will provide the missing nutritional links for most dietary supplements. Nitrogen balance is Collagen is a fibrous protein originally present in the maintained for the support of age related collagen loss and cartilage damage. It is an excellent product for those with a body, which in combination with hyaluronic acid, is a sedentary lifestyle who may suffer from repetitive joint pain or strong element for keeping a moisturized and smooth discomfort skin. Collagen is a natural substance in our body which > This drink will make you a pleasant and helps maintain healthy decreases with age. Moreover, collagen is a key element Joints, hair, skin, nails and lifestyle. in the health of joints, cartilage, tendons, bones and all connective human tissue. Recommended for > For all men and women wanting to have supplement to provide the body with nutrients required to help maintain joint mobility and a healthy lifestyle -

Collagen and Elastin Fibres
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.49 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 49-58 Collagen and elastin fibres A. J. BAILEY From the Agricultural Research Council, Meat Research Institute, Langford, Bristol Although an understanding of the intracellular native collagen was generated from type I pro- biosynthesis of both collagen and elastin is of collagen. Whether this means that the two pro- considerable importance it is the subsequent extra- collagens are converted by different enzyme systems cellular changes involving fibrogenesis and cross- and the type III enzyme was deficient in these linking that ensure that these proteins ultimately fibroblast cultures, or that the processing of pro become the major supporting tissues of the body. type III is extremely slow, is not known. The latter This paper summarises the formation and stability proposal is consistent with the higher proportion of collagen and elastin fibres. of soluble pro type III extractable from tissue (Lenaers and Lapiere, 1975; Timpl et al., 1975). Collagen Basement membrane collagens, on the other hand, do not form fibres and this property may be The non-helical regions at the ends of the triple due to the retention of the non-helical extension helix of procollagen probably provide a number of peptides (Kefalides, 1973). In-vivo biosynthetic different intracellular functions-that is, initiating studies showing the absence of any extension peptide rapid formation of the triple helix; inhibiting intra- removal support this (Minor et al., 1976), but other cellular fibrillogenesis; and facilitating transmem- workers have reported that there is some cleavage brane movement. -

Effect of Collagen Hydrolysates from Silver Carp Skin (Hypophthalmichthys Molitrix) on Osteoporosis in Chronologically Aged Mice: Increasing Bone Remodeling
nutrients Article Effect of Collagen Hydrolysates from Silver Carp Skin (Hypophthalmichthys molitrix) on Osteoporosis in Chronologically Aged Mice: Increasing Bone Remodeling Ling Zhang 1, Siqi Zhang 1, Hongdong Song 1 and Bo Li 1,2,* 1 Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China; [email protected] (L.Z.); [email protected] (S.Z.); [email protected] (H.S.) 2 Beijing Higher Institution Engineering Research Center of Animal Product, Beijing 100083, China * Correspondence: [email protected]; Tel./Fax: +86-10-6273-7669 Received: 15 August 2018; Accepted: 30 September 2018; Published: 4 October 2018 Abstract: Osteoporosis is a common skeletal disorder in humans and gelatin hydrolysates from mammals have been reported to improve osteoporosis. In this study, 13-month-old mice were used to evaluate the effects of collagen hydrolysates (CHs) from silver carp skin on osteoporosis. No significant differences were observed in mice body weight, spleen or thymus indices after daily intake of antioxidant collagen hydrolysates (ACH; 200 mg/kg body weight (bw) (LACH), 400 mg/kg bw (MACH), 800 mg/kg bw (HACH)), collagenase hydrolyzed collagen hydrolysates (CCH) or proline (400 mg/kg body weight) for eight weeks, respectively. ACH tended to improve bone mineral density, increase bone hydroxyproline content, enhance alkaline phosphatase (ALP) level and reduce tartrate-resistant acid phosphatase 5b (TRAP-5b) activity in serum, with significant differences observed between the MACH and model groups (p < 0.05). ACH exerted a better effect on osteoporosis than CCH at the identical dose, whereas proline had no significant effect on repairing osteoporosis compared to the model group. -

Ideal Chain Conformations and Statistics
ENAS 606 : Polymer Physics Chinedum Osuji 01.24.2013:HO2 Ideal Chain Conformations and Statistics 1 Overview Consideration of the structure of macromolecules starts with a look at the details of chain level chemical details which can impact the conformations adopted by the polymer. In the case of saturated carbon ◦ backbones, while maintaining the desired Ci−1 − Ci − Ci+1 bond angle of 112 , the placement of the final carbon in the triad above can occur at any point along the circumference of a circle, defining a torsion angle '. We can readily recognize the energetic differences as a function this angle, U(') such that there are 3 minima - a deep minimum corresponding the the trans state, for which ' = 0 and energetically equivalent gauche− and gauche+ states at ' = ±120 degrees, as shown in Fig 1. Figure 1: Trans, gauche- and gauche+ configurations, and their energetic sates 1.1 Static Flexibility The static flexibility of the chain in equilibrium is determined by the difference between the levels of the energy minima corresponding the gauche and trans states, ∆. If ∆ < kT , the g+, g− and t states occur with similar probability, and so the chain can change direction and appears as a random coil. If ∆ takes on a larger value, then the t conformations will be enriched, so the chain will be rigid locally, but on larger length scales, the eventual occurrence of g+ and g− conformations imparts a random conformation. Overall, if we ignore details on some length scale smaller than lp, the persistence length, the polymer appears as a continuous flexible chain where 1 lp = l0 exp(∆/kT ) (1) where l0 is something like a monomer length. -

Evaluation of Elastin/Collagen Content in Human Dermis In-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging
Cosmetics 2014, 1, 211-221; doi:10.3390/cosmetics1030211 OPEN ACCESS cosmetics ISSN 2079-9284 www.mdpi.com/journal/cosmetics Article Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging Jean-Christophe Pittet 1,*, Olga Freis 2,†, Marie-Danielle Vazquez-Duchêne 2,†, Gilles Périé 2,† and Gilles Pauly 2,† 1 Orion Concept, 100 Rue de Suède, 37100 Tours, France 2 BASF Beauty Care Solutions France SAS, 3 Rue de Seichamps, CS 71040 Pulnoy, 54272 Essey-lès-Nancy Cedex, France; E-Mails: [email protected] (O.F.); [email protected] (M.-D.V.-D.); [email protected] (G.Pé.); [email protected] (G.Pa.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-247-052-316; Fax: +33-610-786-695. Received: 14 March 2014; in revised form: 31 July 2014 / Accepted: 1 August 2014 / Published: 20 August 2014 Abstract: The aim of this study was to evaluate the influence of the depth of the dermis on the measured collagen and elastin levels and to establish the correlation between the amount of these two extracellular matrix (ECM) components and age. Multiphoton Microscopy (MPM) that measures the autofluorescence (AF) and second harmonic generation (SHG) was used to quantify the levels of elastin and collagen and to determine the SAAID (SHG-to-AF Aging Index of Dermis) at two different skin depths. A 50 MHz ultrasound scanner was used for the calculation of the Sub Epidermal Non Echogenic Band (SENEB). -

Native-Like Mean Structure in the Unfolded Ensemble of Small Proteins
B doi:10.1016/S0022-2836(02)00888-4 available online at http://www.idealibrary.com on w J. Mol. Biol. (2002) 323, 153–164 Native-like Mean Structure in the Unfolded Ensemble of Small Proteins Bojan Zagrovic1, Christopher D. Snow1, Siraj Khaliq2 Michael R. Shirts2 and Vijay S. Pande1,2* 1Biophysics Program The nature of the unfolded state plays a great role in our understanding of Stanford University, Stanford proteins. However, accurately studying the unfolded state with computer CA 94305-5080, USA simulation is difficult, due to its complexity and the great deal of sampling required. Using a supercluster of over 10,000 processors we 2Department of Chemistry have performed close to 800 ms of molecular dynamics simulation in Stanford University, Stanford atomistic detail of the folded and unfolded states of three polypeptides CA 94305-5080, USA from a range of structural classes: the all-alpha villin headpiece molecule, the beta hairpin tryptophan zipper, and a designed alpha-beta zinc finger mimic. A comparison between the folded and the unfolded ensembles reveals that, even though virtually none of the individual members of the unfolded ensemble exhibits native-like features, the mean unfolded structure (averaged over the entire unfolded ensemble) has a native-like geometry. This suggests several novel implications for protein folding and structure prediction as well as new interpretations for experiments which find structure in ensemble-averaged measurements. q 2002 Elsevier Science Ltd. All rights reserved Keywords: mean-structure hypothesis; unfolded state of proteins; *Corresponding author distributed computing; conformational averaging Introduction under folding conditions, with some notable exceptions.14 – 16 This is understandable since under Historically, the unfolded state of proteins has such conditions the unfolded state is an unstable, received significantly less attention than the folded fleeting species making any kind of quantitative state.1 The reasons for this are primarily its struc- experimental measurement very difficult. -
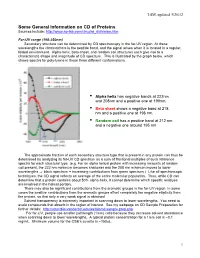
CD of Proteins Sources Include
LSM, updated 3/26/12 Some General Information on CD of Proteins Sources include: http://www.ap-lab.com/circular_dichroism.htm Far-UV range (190-250nm) Secondary structure can be determined by CD spectroscopy in the far-UV region. At these wavelengths the chromophore is the peptide bond, and the signal arises when it is located in a regular, folded environment. Alpha-helix, beta-sheet, and random coil structures each give rise to a characteristic shape and magnitude of CD spectrum. This is illustrated by the graph below, which shows spectra for poly-lysine in these three different conformations. • Alpha helix has negative bands at 222nm and 208nm and a positive one at 190nm. • Beta sheet shows a negative band at 218 nm and a positive one at 196 nm. • Random coil has a positive band at 212 nm and a negative one around 195 nm. The approximate fraction of each secondary structure type that is present in any protein can thus be determined by analyzing its far-UV CD spectrum as a sum of fractional multiples of such reference spectra for each structural type. (e.g. For an alpha helical protein with increasing amounts of random coil present, the 222 nm minimum becomes shallower and the 208 nm minimum moves to lower wavelengths ⇒ black spectrum + increasing contributions from green spectrum.) Like all spectroscopic techniques, the CD signal reflects an average of the entire molecular population. Thus, while CD can determine that a protein contains about 50% alpha-helix, it cannot determine which specific residues are involved in the helical portion. -

Blood Vitronectin Is a Major Activator of LIF and IL-6 in the Brain Through Integrin–FAK and Upar Signaling Matthew P
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs202580. doi:10.1242/jcs.202580 RESEARCH ARTICLE Blood vitronectin is a major activator of LIF and IL-6 in the brain through integrin–FAK and uPAR signaling Matthew P. Keasey1, Cuihong Jia1, Lylyan F. Pimentel1,2, Richard R. Sante1, Chiharu Lovins1 and Theo Hagg1,* ABSTRACT Microglia and astrocytes express the VTN receptors αvβ3 and α β We defined how blood-derived vitronectin (VTN) rapidly and potently v 5 integrin (Herrera-Molina et al., 2012; Kang et al., 2008; activates leukemia inhibitory factor (LIF) and pro-inflammatory Milner, 2009; Welser-Alves et al., 2011). Microglia and astrocytes, interleukin 6 (IL-6) in vitro and after vascular injury in the brain. as well as endothelial cells, are major producers of pro- α in vitro Treatment with VTN (but not fibrinogen, fibronectin, laminin-111 or inflammatory cytokines, such as IL-6 and TNF , and collagen-I) substantially increased LIF and IL-6 within 4 h in after traumatic or ischemic injury to the brain (Banner et al., 1997; C6-astroglioma cells, while VTN−/− mouse plasma was less effective Erta et al., 2012; Lau and Yu, 2001) or upon self-induction by IL-6 than that from wild-type mice. LIF and IL-6 were induced by (Van Wagoner and Benveniste, 1999). IL-6 is a major regulator of a intracerebral injection of recombinant human (rh)VTN in mice, but variety of inflammatory disorders and a target for therapies (Hunter induction seen upon intracerebral hemorrhage was less in VTN−/− and Jones, 2015). -

COLLAGEN·NATIVE·TYPE 2 Frequently Asked Questions
COLLAGEN·NATIVE·TYPE 2 Frequently Asked Questions Why “NATIVE”? What are the benefits of native type II collagen? A protein is a three-dimensional molecule with a specific shape, Native type II collagen ofers the same benefits as collagen and this shape allows it to carry out its specific role in the body. peptides—they both provide collagen building blocks to restore While the shapes of some proteins cause chemical reactions or the body’s supply. But, native type II collagen ofers even more relay signals, the helical shape of collagen provides structural health advantages. support in tissues such as skin and cartilage. Improved Skin Health: Collagen is the most abundant protein We use the terms “native” or “undenatured” to describe proteins, in the second layer of skin, and its strand-like molecules link including collagen, that are still in their natural three- together to give structural support to the skin. While age-related dimensional shapes. While other collagen supplements contain collagen loss can lead to wrinkle formation, collagen supple- proteins that have been broken into fragments, or “peptides,” mentation can both reduce the appearance of wrinkles already COLLAGEN•NATIVE•TYPE 2 is extracted gently to ensure that present and protect against further wrinkle formation. Native the collagen maintains its helical shape. Once ingested, the type II collagen is particularly efective at stimulating collagen native collagen is broken down by the body’s digestive system production to improve skin health and appearance, and it also and then used to support collagen production in the body. promotes healing at sites of tissue damage and inflammation. -

With Caviar, Keratin & Collagen
WITH CAVIAR, KERATIN & COLLAGEN Professional treatments for colour, bleaching, care and maintenance with CAVIAR, KERATIN and COLLAGEN Professional styling products with CAVIAR, KERATIN and COLLAGEN Technical professional products with CAVIAR, KERATIN and COLLAGEN 2 Professional products by Very high technology professional products to colour, treat and protect hair from the continuous chemical and environmental stress caused on a daily basis. Formulas based on: Caviar Keratin Collagen 3 WITH CAVIAR, KERATIN & COLLAGEN Colouring permanentCream professional ammonia PPD • Respects the hair structure thanks to an exposure free free time of 12 minutes • Non-progressive • Ammonia free • Paraphenylenediamine free • Unleashes all the EFFICANCY of its active principles and maximum COLOURING POWER Mix 1 : 1 4 WITH CAVIAR, KERATIN & COLLAGEN 1. Respect for scalp and hair thanks to a shorter processing time 2. Maximum grey hair coverage 3. Lightens up to 4 tones 4. Ammonia free and Paraphenylenediamine free 5. Safe application even on customers with a sensitive scalp 6. Extreme colour brilliancy and uniformity 7. Very easy and practical to use 8. Long lasting reflections 9. High colour fastness 10. Great protection action 11. Effective restructuring action benefits 12. Maximum conditioning 5 WITH CAVIAR, KERATIN & COLLAGEN Benefits 1 2 3 Respect for scalp and hair thanks to a shorter processing Lightens up to Maximum grey hair 4 tones time coverage 6 WITH CAVIAR, KERATIN & COLLAGEN has been developed according Cosmetic colour pDT BASE Be Colour 12 Minute Dpe DIAMINOPHENOXYETHANOL to the rules of the “molar stoichiometry,” a technique creamy gel with RESORCINOL m-AMINOPHENOL of colouring clear and uncompromising. It is based on CAVIAR, a mathematical principle according to which the molar concentration of the dye base is equal to the molar KERATIN and concentration of the sum of the other colouring couplers. -

Effects of Collagen-Derived Bioactive Peptides and Natural Antioxidant
www.nature.com/scientificreports OPEN Efects of collagen-derived bioactive peptides and natural antioxidant compounds on Received: 29 December 2017 Accepted: 19 June 2018 proliferation and matrix protein Published: xx xx xxxx synthesis by cultured normal human dermal fbroblasts Suzanne Edgar1, Blake Hopley1, Licia Genovese2, Sara Sibilla2, David Laight1 & Janis Shute1 Nutraceuticals containing collagen peptides, vitamins, minerals and antioxidants are innovative functional food supplements that have been clinically shown to have positive efects on skin hydration and elasticity in vivo. In this study, we investigated the interactions between collagen peptides (0.3–8 kDa) and other constituents present in liquid collagen-based nutraceuticals on normal primary dermal fbroblast function in a novel, physiologically relevant, cell culture model crowded with macromolecular dextran sulphate. Collagen peptides signifcantly increased fbroblast elastin synthesis, while signifcantly inhibiting release of MMP-1 and MMP-3 and elastin degradation. The positive efects of the collagen peptides on these responses and on fbroblast proliferation were enhanced in the presence of the antioxidant constituents of the products. These data provide a scientifc, cell-based, rationale for the positive efects of these collagen-based nutraceutical supplements on skin properties, suggesting that enhanced formation of stable dermal fbroblast-derived extracellular matrices may follow their oral consumption. Te biophysical properties of the skin are determined by the interactions between cells, cytokines and growth fac- tors within a network of extracellular matrix (ECM) proteins1. Te fbril-forming collagen type I is the predomi- nant collagen in the skin where it accounts for 90% of the total and plays a major role in structural organisation, integrity and strength2.