Collagen of the Extracellular Matrix. Functional and Structural Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Collagen and Elastin Fibres
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.49 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 49-58 Collagen and elastin fibres A. J. BAILEY From the Agricultural Research Council, Meat Research Institute, Langford, Bristol Although an understanding of the intracellular native collagen was generated from type I pro- biosynthesis of both collagen and elastin is of collagen. Whether this means that the two pro- considerable importance it is the subsequent extra- collagens are converted by different enzyme systems cellular changes involving fibrogenesis and cross- and the type III enzyme was deficient in these linking that ensure that these proteins ultimately fibroblast cultures, or that the processing of pro become the major supporting tissues of the body. type III is extremely slow, is not known. The latter This paper summarises the formation and stability proposal is consistent with the higher proportion of collagen and elastin fibres. of soluble pro type III extractable from tissue (Lenaers and Lapiere, 1975; Timpl et al., 1975). Collagen Basement membrane collagens, on the other hand, do not form fibres and this property may be The non-helical regions at the ends of the triple due to the retention of the non-helical extension helix of procollagen probably provide a number of peptides (Kefalides, 1973). In-vivo biosynthetic different intracellular functions-that is, initiating studies showing the absence of any extension peptide rapid formation of the triple helix; inhibiting intra- removal support this (Minor et al., 1976), but other cellular fibrillogenesis; and facilitating transmem- workers have reported that there is some cleavage brane movement. -

Evaluation of Elastin/Collagen Content in Human Dermis In-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging
Cosmetics 2014, 1, 211-221; doi:10.3390/cosmetics1030211 OPEN ACCESS cosmetics ISSN 2079-9284 www.mdpi.com/journal/cosmetics Article Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging Jean-Christophe Pittet 1,*, Olga Freis 2,†, Marie-Danielle Vazquez-Duchêne 2,†, Gilles Périé 2,† and Gilles Pauly 2,† 1 Orion Concept, 100 Rue de Suède, 37100 Tours, France 2 BASF Beauty Care Solutions France SAS, 3 Rue de Seichamps, CS 71040 Pulnoy, 54272 Essey-lès-Nancy Cedex, France; E-Mails: [email protected] (O.F.); [email protected] (M.-D.V.-D.); [email protected] (G.Pé.); [email protected] (G.Pa.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-247-052-316; Fax: +33-610-786-695. Received: 14 March 2014; in revised form: 31 July 2014 / Accepted: 1 August 2014 / Published: 20 August 2014 Abstract: The aim of this study was to evaluate the influence of the depth of the dermis on the measured collagen and elastin levels and to establish the correlation between the amount of these two extracellular matrix (ECM) components and age. Multiphoton Microscopy (MPM) that measures the autofluorescence (AF) and second harmonic generation (SHG) was used to quantify the levels of elastin and collagen and to determine the SAAID (SHG-to-AF Aging Index of Dermis) at two different skin depths. A 50 MHz ultrasound scanner was used for the calculation of the Sub Epidermal Non Echogenic Band (SENEB). -

Collagen VI-Related Myopathy
Collagen VI-related myopathy Description Collagen VI-related myopathy is a group of disorders that affect skeletal muscles (which are the muscles used for movement) and connective tissue (which provides strength and flexibility to the skin, joints, and other structures throughout the body). Most affected individuals have muscle weakness and joint deformities called contractures that restrict movement of the affected joints and worsen over time. Researchers have described several forms of collagen VI-related myopathy, which range in severity: Bethlem myopathy is the mildest, an intermediate form is moderate in severity, and Ullrich congenital muscular dystrophy is the most severe. People with Bethlem myopathy usually have loose joints (joint laxity) and weak muscle tone (hypotonia) in infancy, but they develop contractures during childhood, typically in their fingers, wrists, elbows, and ankles. Muscle weakness can begin at any age but often appears in childhood to early adulthood. The muscle weakness is slowly progressive, with about two-thirds of affected individuals over age 50 needing walking assistance. Older individuals may develop weakness in respiratory muscles, which can cause breathing problems. Some people with this mild form of collagen VI-related myopathy have skin abnormalities, including small bumps called follicular hyperkeratosis on the arms and legs; soft, velvety skin on the palms of the hands and soles of the feet; and abnormal wound healing that creates shallow scars. The intermediate form of collagen VI-related myopathy is characterized by muscle weakness that begins in infancy. Affected children are able to walk, although walking becomes increasingly difficult starting in early adulthood. They develop contractures in the ankles, elbows, knees, and spine in childhood. -

The Beneficial Regulation of Extracellular Matrix
cosmetics Article The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts Neena Philips *, Xinxing Ding, Pranathi Kandalai, Ilonka Marte, Hunter Krawczyk and Richard Richardson School of Natural Sciences, Fairleigh Dickinson University, Teaneck, NJ 07601, USA * Correspondence: [email protected] or [email protected] Received: 30 June 2019; Accepted: 20 July 2019; Published: 1 August 2019 Abstract: Intrinsic skin aging and photoaging, from exposure to ultraviolet (UV) radiation, are associated with altered regulation of genes associated with the extracellular matrix (ECM) and inflammation, as well as cellular damage from oxidative stress. The regulatory properties of 1α, 25dihydroxyvitamin D3 (vitamin D) include endocrine, ECM regulation, cell differentiation, photoprotection, and anti-inflammation. The goal of this research was to identify the beneficial effects of vitamin D in preventing intrinsic skin aging and photoaging, through its direct effects as well as its effects on the ECM, associated heat shock proteins (HSP-47, and -70), cellular oxidative stress effects, and inflammatory cytokines [interleukin (IL)-1 and IL-8] in non-irradiated, UVA-radiated, UVB-radiated dermal fibroblasts. With regard to the ECM, vitamin D stimulated type I collagen and inhibited cellular elastase activity in non-irradiated fibroblasts; and stimulated type I collagen and HSP-47, and inhibited elastin expression and elastase activity in UVA-radiated dermal fibroblasts. With regard to cellular protection, vitamin D inhibited oxidative damage to DNA, RNA, and lipids in non-irradiated, UVA-radiated and UVB-radiated fibroblasts, and, in addition, increased cell viability of UVB-radiated cells. -

Dietary Protein Restriction Rapidly Reduces Transforming Growth Factor
Proc. Natl. Acad. Sci. USA Vol. 88, pp. 9765-9769, November 1991 Medical Sciences Dietary protein restriction rapidly reduces transforming growth factor p1 expression in experimental glomerulonephritis (extraceliular matrix/transforming growth factor 8/glomerulonephritis) SEIYA OKUDA*, TAKAMICHI NAKAMURA*, TATSUO YAMAMOTO*, ERKKI RUOSLAHTIt, AND WAYNE A. BORDER*t *Division of Nephrology, University of Utah School of Medicine, Salt Lake City, UT 84132; and tCancer Research Center, La Jolla Cancer Research Foundation, La Jolla, CA 92037 Communicated by Eugene Roberts, August 19, 1991 (receivedfor review April 29, 1991) ABSTRACT Dietary protein restriction has been shown to TGF-/31 on both cell types is to regulate the synthesis of two slow the rate of loss of kidney function in humans with chondroitin/dermatan sulfate proteoglycans, biglycan and progressive glomerulosclerosis due to glomerulonephritis or decorin, both of which can bind TGF-P1 (23). In an experi- diabetes mellitus. A central feature of glomerulosclerosis is the mental model ofglomerulonephritis in the rat, we have found pathological accumulation of extracellular matrix within the a close association between elevated expression of the diseased glomeruli. Transforming growth factor j1 (TGF-.81) TGF-131 gene and the development of glomerulonephritis is known to have widespread regulatory effects on extracellular (10). Seven days after glomerular injury, at the time of matrix and has been implicated as a major cause of increased significant extracellular matrix accumulation, the glomeruli extracellular matrix synthesis and buildup of pathological showed a 5-fold increase in TGF-f31 mRNA and a nearly matrix within glomeruli in experimental glomerulonephritis. 50-fold increase in production ofbiglycan and decorin. -

Extracellular Matrix Grafts: from Preparation to Application (Review)
INTERNATIONAL JOURNAL OF MOleCular meDICine 47: 463-474, 2021 Extracellular matrix grafts: From preparation to application (Review) YONGSHENG JIANG1*, RUI LI1,2*, CHUNCHAN HAN1 and LIJIANG HUANG1 1Science and Education Management Center, The Affiliated Xiangshan Hospital of Wenzhou Medical University, Ningbo, Zhejiang 315700; 2School of Chemistry, Sun Yat-sen University, Guangzhou, Guangdong 510275, P.R. China Received July 30, 2020; Accepted December 3, 2020 DOI: 10.3892/ijmm.2020.4818 Abstract. Recently, the increasing emergency of traffic acci- Contents dents and the unsatisfactory outcome of surgical intervention are driving research to seek a novel technology to repair trau- 1. Introduction matic soft tissue injury. From this perspective, decellularized 2. ECM-G characterization matrix grafts (ECM-G) including natural ECM materials, and 3. Methods of decellularization treatments their prepared hydrogels and bioscaffolds, have emerged as 4. Removal of residual cellular components and chemicals possible alternatives for tissue engineering and regenerative 5. Application of ECM-P in regenerative medicine medicine. Over the past decades, several physical and chemical 6. Challenges and future outlook on ECM-P decellularization methods have been used extensively to deal 7. Conclusions with different tissues/organs in an attempt to carefully remove cellular antigens while maintaining the non-immunogenic ECM components. It is anticipated that when the decellular- 1. Introduction ized biomaterials are seeded with cells in vitro or incorporated into irregularly shaped defects in vivo, they can provide the The extracellular matrix (ECM) derived from organs/tissues is appropriate biomechanical and biochemical conditions for a complex, highly organized assembly of macromolecules with directing cell behavior and tissue remodeling. -

Blood Vitronectin Is a Major Activator of LIF and IL-6 in the Brain Through Integrin–FAK and Upar Signaling Matthew P
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs202580. doi:10.1242/jcs.202580 RESEARCH ARTICLE Blood vitronectin is a major activator of LIF and IL-6 in the brain through integrin–FAK and uPAR signaling Matthew P. Keasey1, Cuihong Jia1, Lylyan F. Pimentel1,2, Richard R. Sante1, Chiharu Lovins1 and Theo Hagg1,* ABSTRACT Microglia and astrocytes express the VTN receptors αvβ3 and α β We defined how blood-derived vitronectin (VTN) rapidly and potently v 5 integrin (Herrera-Molina et al., 2012; Kang et al., 2008; activates leukemia inhibitory factor (LIF) and pro-inflammatory Milner, 2009; Welser-Alves et al., 2011). Microglia and astrocytes, interleukin 6 (IL-6) in vitro and after vascular injury in the brain. as well as endothelial cells, are major producers of pro- α in vitro Treatment with VTN (but not fibrinogen, fibronectin, laminin-111 or inflammatory cytokines, such as IL-6 and TNF , and collagen-I) substantially increased LIF and IL-6 within 4 h in after traumatic or ischemic injury to the brain (Banner et al., 1997; C6-astroglioma cells, while VTN−/− mouse plasma was less effective Erta et al., 2012; Lau and Yu, 2001) or upon self-induction by IL-6 than that from wild-type mice. LIF and IL-6 were induced by (Van Wagoner and Benveniste, 1999). IL-6 is a major regulator of a intracerebral injection of recombinant human (rh)VTN in mice, but variety of inflammatory disorders and a target for therapies (Hunter induction seen upon intracerebral hemorrhage was less in VTN−/− and Jones, 2015). -

With Caviar, Keratin & Collagen
WITH CAVIAR, KERATIN & COLLAGEN Professional treatments for colour, bleaching, care and maintenance with CAVIAR, KERATIN and COLLAGEN Professional styling products with CAVIAR, KERATIN and COLLAGEN Technical professional products with CAVIAR, KERATIN and COLLAGEN 2 Professional products by Very high technology professional products to colour, treat and protect hair from the continuous chemical and environmental stress caused on a daily basis. Formulas based on: Caviar Keratin Collagen 3 WITH CAVIAR, KERATIN & COLLAGEN Colouring permanentCream professional ammonia PPD • Respects the hair structure thanks to an exposure free free time of 12 minutes • Non-progressive • Ammonia free • Paraphenylenediamine free • Unleashes all the EFFICANCY of its active principles and maximum COLOURING POWER Mix 1 : 1 4 WITH CAVIAR, KERATIN & COLLAGEN 1. Respect for scalp and hair thanks to a shorter processing time 2. Maximum grey hair coverage 3. Lightens up to 4 tones 4. Ammonia free and Paraphenylenediamine free 5. Safe application even on customers with a sensitive scalp 6. Extreme colour brilliancy and uniformity 7. Very easy and practical to use 8. Long lasting reflections 9. High colour fastness 10. Great protection action 11. Effective restructuring action benefits 12. Maximum conditioning 5 WITH CAVIAR, KERATIN & COLLAGEN Benefits 1 2 3 Respect for scalp and hair thanks to a shorter processing Lightens up to Maximum grey hair 4 tones time coverage 6 WITH CAVIAR, KERATIN & COLLAGEN has been developed according Cosmetic colour pDT BASE Be Colour 12 Minute Dpe DIAMINOPHENOXYETHANOL to the rules of the “molar stoichiometry,” a technique creamy gel with RESORCINOL m-AMINOPHENOL of colouring clear and uncompromising. It is based on CAVIAR, a mathematical principle according to which the molar concentration of the dye base is equal to the molar KERATIN and concentration of the sum of the other colouring couplers. -

Effects of Collagen-Derived Bioactive Peptides and Natural Antioxidant
www.nature.com/scientificreports OPEN Efects of collagen-derived bioactive peptides and natural antioxidant compounds on Received: 29 December 2017 Accepted: 19 June 2018 proliferation and matrix protein Published: xx xx xxxx synthesis by cultured normal human dermal fbroblasts Suzanne Edgar1, Blake Hopley1, Licia Genovese2, Sara Sibilla2, David Laight1 & Janis Shute1 Nutraceuticals containing collagen peptides, vitamins, minerals and antioxidants are innovative functional food supplements that have been clinically shown to have positive efects on skin hydration and elasticity in vivo. In this study, we investigated the interactions between collagen peptides (0.3–8 kDa) and other constituents present in liquid collagen-based nutraceuticals on normal primary dermal fbroblast function in a novel, physiologically relevant, cell culture model crowded with macromolecular dextran sulphate. Collagen peptides signifcantly increased fbroblast elastin synthesis, while signifcantly inhibiting release of MMP-1 and MMP-3 and elastin degradation. The positive efects of the collagen peptides on these responses and on fbroblast proliferation were enhanced in the presence of the antioxidant constituents of the products. These data provide a scientifc, cell-based, rationale for the positive efects of these collagen-based nutraceutical supplements on skin properties, suggesting that enhanced formation of stable dermal fbroblast-derived extracellular matrices may follow their oral consumption. Te biophysical properties of the skin are determined by the interactions between cells, cytokines and growth fac- tors within a network of extracellular matrix (ECM) proteins1. Te fbril-forming collagen type I is the predomi- nant collagen in the skin where it accounts for 90% of the total and plays a major role in structural organisation, integrity and strength2. -
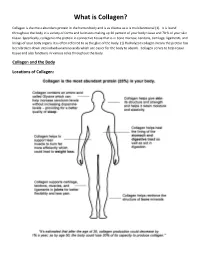
What Is Collagen?
What is Collagen? Collagen is the most abundant protein in the human body and is as diverse as it is multifunctional (1). It is found throughout the body in a variety of forms and functions making up 30 percent of your body tissue and 70 % of your skin tissue. Specifically, collagen is the protein in connective tissue that is in bone marrow, tendons, cartilage, ligaments, and linings of your body organs. It is often referred to as the glue of the body. (1) Hydrolyzed collagen means the protein has been broken down into individual amino acids which are easier for the body to absorb. Collagen serves to help repair tissue and also functions in various roles throughout the body. Collagen and the Body Locations of Collagen: What Does Collagen Do? Collagen has numerous structural properties but also plays a vital role in the repair of almost all the body’s tissues. Some diseases are directly linked to lacking this essential protein. Depending on which part of the body it is located, collagen serves different purposes. In skin: Found in the inner layer, this connective tissue gives the skin its structure and strength and also functions in the replacement of dead skin cells. A lack of collagen in the skin can contribute to a decrease in skin health leading to stretch marks, dark spots, and infections as well as affecting the skin’s ability to maintain moisture. In internal organs and blood vessels: In the lining of your organs like in the stomach, kidneys, blood vessels and spleen, collagen functions as a protective covering and a fibrous barrier. -

Empowering Collagen Targeting for Diagnosis and Treatment of Human Conditions
EmpoweringEmpowering collagen collagen targetingtargeting for for diagnosis prognosis of fibrotic and treatment of Manka,SW. 2012 conditions.human conditions. 1 3Helix as a platform diagnostic company 2020 2022 2030 Innovative research reagent for Clinic histopathology providing Platform fibrotic prognostic detection of collagen damage best in class prognostic ability in within multi-disease states liver fibrosis (NAFLD, NASH) Strengthening IP portfolio with market Clinical histopathology AND Non-Invasive approaches serum testing and medical disrupting products while subsequently Analytic specific reagent to allow for fast imaging developing strong partnerships with world market access leading companies for commercialization Targeting impactful markets of IPF, kidney Focused on paired biopsy research and fibrosis, AMD, Keloids and cardiac fibrosis collaboration with clinical laboratories. in addition to fibrotic liver diseases. 2 Liver Fibrosis market is GROWING Fatty Liver Fibrotic Liver Healthy Liver Cirrhosis NAFLD NASH USA Epidemiology 328 Million 83 Million 16 Million 1.5 Million (2015) Predicted USA Epidemiology 360 Million 101 Million 27 Million 3.4 Million (2030) Estes,C. 2018 3 NASH Therapeutics are finishing clinical trials and are coming to market. • VK2809 Phase II • OCALIVA (OCA) • NDA Filed • $78,000/year current cost • $20,000 predicted • Resmetirom Phase III • 23% respond to treatment 16 Million NASH patients in the USA * $20,000 • $320 Billion Annual Cost for treatable market Aramchol Phase III/IV 4 Stratification of patient population is needed to reduce unnecessary therapeutic intervention. • Progression of NAFL and 100% NASH is variable patient to NAFLD patient. 33% • Prediction of the progression can modify the Fibrosis Progression 20% disease intervention and treatment. Rapid Fibrosis • No product on the market Progression (stage 0 to stage today is equipped for 3/4 over 5.9 years) prognosis of liver fibrosis Singh, S. -

Tendon Extracellular Matrix Remodeling and Defective Cell Polarization in the Presence of Collagen VI Mutations
cells Article Tendon Extracellular Matrix Remodeling and Defective Cell Polarization in the Presence of Collagen VI Mutations Manuela Antoniel 1,2, Francesco Traina 3,4, Luciano Merlini 5 , Davide Andrenacci 1,2, Domenico Tigani 6, Spartaco Santi 1,2, Vittoria Cenni 1,2, Patrizia Sabatelli 1,2,*, Cesare Faldini 7 and Stefano Squarzoni 1,2 1 CNR-Institute of Molecular Genetics “Luigi Luca Cavalli-Sforza”-Unit of Bologna, 40136 Bologna, Italy; [email protected] (M.A.); [email protected] (D.A.); [email protected] (S.S.); [email protected] (V.C.); [email protected] (S.S.) 2 IRCCS Istituto Ortopedico Rizzoli, 40136 Bologna, Italy 3 Ortopedia-Traumatologia e Chirurgia Protesica e dei Reimpianti d’Anca e di Ginocchio, Istituto Ortopedico Rizzoli di Bologna, 40136 Bologna, Italy; [email protected] 4 Dipartimento di Scienze Biomediche, Odontoiatriche e delle Immagini Morfologiche e Funzionali, Università Degli Studi Di Messina, 98122 Messina, Italy 5 Department of Biomedical and Neuromotor Sciences, University of Bologna, 40123 Bologna, Italy; [email protected] 6 Department of Orthopedic and Trauma Surgery, Ospedale Maggiore, 40133 Bologna, Italy; [email protected] 7 1st Orthopaedic and Traumatologic Clinic, IRCCS Istituto Ortopedico Rizzoli, 40136 Bologna, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-051-6366755; Fax: +39-051-4689922 Received: 20 December 2019; Accepted: 7 February 2020; Published: 11 February 2020 Abstract: Mutations in collagen VI genes cause two major clinical myopathies, Bethlem myopathy (BM) and Ullrich congenital muscular dystrophy (UCMD), and the rarer myosclerosis myopathy. In addition to congenital muscle weakness, patients affected by collagen VI-related myopathies show axial and proximal joint contractures, and distal joint hypermobility, which suggest the involvement of tendon function.