A Novel Antiageing Mechanism for Retinol: Induction of Dermal Elastin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recombinant Laminin Α5 LG1-3 Domains Support the Stemness of Human Mesenchymal Stem Cells
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 166, 2021 Recombinant laminin α5 LG1-3 domains support the stemness of human mesenchymal stem cells SUJIN LEE1*, DONG‑SUNG LEE2* and JUN‑HYEOG JANG1 1Department of Biochemistry, College of Medicine, Inha University, Incheon 22212; 2College of Pharmacy, Chosun University, Gwangju 61452, Republic of Korea Received April 23, 2020; Accepted November 24, 2020 DOI: 10.3892/etm.2020.9597 Abstract. The extracellular matrix components laminin and be met by mimicking the in vivo extracellular matrix (ECM) elastin serve key roles in stem cell therapy. Elastin‑like poly‑ configuration, thereby modulating the activity of stem cells peptides (ELPs), derived from a soluble form of elastin, affect in vitro (2). The principle behind this hypothesis is that the the proliferation and differentiation of various types of cells. ECM not only functions as structural support for stem cells In the present study, a novel protein was designed containing in vivo but also provides biochemical cues for their mainte‑ globular domains 1‑3 of laminin α5 (Lα5LG1‑3) fused to nance versus directed differentiation (3). ELPs (Lα5LG1‑3/ELP). Lα5LG1‑3/ELP was expressed in Basement membranes (BMs) are a subgroup of the ECM Escherichia coli and displayed a molecular size of ~70 kDa that is necessary for cell differentiation during early devel‑ on 12% SDS‑polyacrylamide gels. The cellular activities, opmental processes. In addition, BMs are critical for the such as cellular adhesion (adhesion assay) and proliferation formation and maintenance of mature tissues (4,5). Laminin, (MTT cytotoxicity assay), of human mesenchymal stem one of the components of BMs, consists of three genetically cells (hMSCs) treated with 1 µg/ml of Lα5LG1‑3/ELP were distinct subunits called α, β and γ chains, which are assembled enhanced compared with those of untreated cells. -

Collagen and Elastin Fibres
J Clin Pathol: first published as 10.1136/jcp.s3-12.1.49 on 1 January 1978. Downloaded from J. clin. Path., 31, Suppl. (Roy. Coll. Path.), 12, 49-58 Collagen and elastin fibres A. J. BAILEY From the Agricultural Research Council, Meat Research Institute, Langford, Bristol Although an understanding of the intracellular native collagen was generated from type I pro- biosynthesis of both collagen and elastin is of collagen. Whether this means that the two pro- considerable importance it is the subsequent extra- collagens are converted by different enzyme systems cellular changes involving fibrogenesis and cross- and the type III enzyme was deficient in these linking that ensure that these proteins ultimately fibroblast cultures, or that the processing of pro become the major supporting tissues of the body. type III is extremely slow, is not known. The latter This paper summarises the formation and stability proposal is consistent with the higher proportion of collagen and elastin fibres. of soluble pro type III extractable from tissue (Lenaers and Lapiere, 1975; Timpl et al., 1975). Collagen Basement membrane collagens, on the other hand, do not form fibres and this property may be The non-helical regions at the ends of the triple due to the retention of the non-helical extension helix of procollagen probably provide a number of peptides (Kefalides, 1973). In-vivo biosynthetic different intracellular functions-that is, initiating studies showing the absence of any extension peptide rapid formation of the triple helix; inhibiting intra- removal support this (Minor et al., 1976), but other cellular fibrillogenesis; and facilitating transmem- workers have reported that there is some cleavage brane movement. -

Immunohistochemical Expression of Tenascin and Elastin In
Clinical Obstetrics, Gynecology and Reproductive Medicine Research Article ISSN: 2059-4828 Immunohistochemical expression of tenascin and elastin in women with pelvic organ prolapse and/or without stress urinary incontinence Ilias Liapis1, Panagiotis Bakas2, Pafiti-Kondi Agatha3, Matrona Frangou-Plemenou4, Charalampos Karachalios5*, Dimos Sioutis6 and Aggelos Liapis2 1Birmingham Women’s and Children’s Hospital, NHS Foundation Trust, Health Education England Midlands and East-West Midlands, Birmingham, England, United Kingdom 2Second Department of Obstetrics and Gynecology, Aretaieio University Hospital, School of Health Sciences, Medical School, National and Kapodistrian University of Athens, Athens, Attica, Greece 3Pathology Laboratory, Aretaieio University Hospital, School of Health Sciences, Medical School, National and Kapodistrian University of Athens, Athens, Attica, Greece 4Microbiology Laboratory, Aretaieio University Hospital, School of Health Sciences, Medical School, National and Kapodistrian University of Athens, Athens, Attica, Greece 5Second Department of Obstetrics and Gynecology, Aretaieio University Hospital, School of Health Sciences, Medical School, National and Kapodistrian University of Athens, Athens, Attica, Greece 6Third Department of Obstetrics and Gynecology, Attikon University Hospital, School of Health Sciences, Medical School, National and Kapodistrian University of Athens, Athens, Attica, Greece Abstract Background and aim: Pelvic organ prolapse (POP) and stress urinary incontinence (SUI) constitute entities of pelvic floor disorders and most often occur simultaneously in the same patient, adversely affecting women’s quality of life. The pathogenesis of pelvic organ prolapse and stress urinary incontinence is not fully understood. The pelvic viscera are maintained in their place thanks to interconnection of levator ani muscles, cardinal and uterosacral ligaments, and pubocervical and rectovaginal fascia. Ligaments and fascia consist mainly of connective tissue. -

Evaluation of Elastin/Collagen Content in Human Dermis In-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging
Cosmetics 2014, 1, 211-221; doi:10.3390/cosmetics1030211 OPEN ACCESS cosmetics ISSN 2079-9284 www.mdpi.com/journal/cosmetics Article Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging Jean-Christophe Pittet 1,*, Olga Freis 2,†, Marie-Danielle Vazquez-Duchêne 2,†, Gilles Périé 2,† and Gilles Pauly 2,† 1 Orion Concept, 100 Rue de Suède, 37100 Tours, France 2 BASF Beauty Care Solutions France SAS, 3 Rue de Seichamps, CS 71040 Pulnoy, 54272 Essey-lès-Nancy Cedex, France; E-Mails: [email protected] (O.F.); [email protected] (M.-D.V.-D.); [email protected] (G.Pé.); [email protected] (G.Pa.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +33-247-052-316; Fax: +33-610-786-695. Received: 14 March 2014; in revised form: 31 July 2014 / Accepted: 1 August 2014 / Published: 20 August 2014 Abstract: The aim of this study was to evaluate the influence of the depth of the dermis on the measured collagen and elastin levels and to establish the correlation between the amount of these two extracellular matrix (ECM) components and age. Multiphoton Microscopy (MPM) that measures the autofluorescence (AF) and second harmonic generation (SHG) was used to quantify the levels of elastin and collagen and to determine the SAAID (SHG-to-AF Aging Index of Dermis) at two different skin depths. A 50 MHz ultrasound scanner was used for the calculation of the Sub Epidermal Non Echogenic Band (SENEB). -

The Beneficial Regulation of Extracellular Matrix
cosmetics Article The Beneficial Regulation of Extracellular Matrix and Heat Shock Proteins, and the Inhibition of Cellular Oxidative Stress Effects and Inflammatory Cytokines by 1α, 25 dihydroxyvitaminD3 in Non-Irradiated and Ultraviolet Radiated Dermal Fibroblasts Neena Philips *, Xinxing Ding, Pranathi Kandalai, Ilonka Marte, Hunter Krawczyk and Richard Richardson School of Natural Sciences, Fairleigh Dickinson University, Teaneck, NJ 07601, USA * Correspondence: [email protected] or [email protected] Received: 30 June 2019; Accepted: 20 July 2019; Published: 1 August 2019 Abstract: Intrinsic skin aging and photoaging, from exposure to ultraviolet (UV) radiation, are associated with altered regulation of genes associated with the extracellular matrix (ECM) and inflammation, as well as cellular damage from oxidative stress. The regulatory properties of 1α, 25dihydroxyvitamin D3 (vitamin D) include endocrine, ECM regulation, cell differentiation, photoprotection, and anti-inflammation. The goal of this research was to identify the beneficial effects of vitamin D in preventing intrinsic skin aging and photoaging, through its direct effects as well as its effects on the ECM, associated heat shock proteins (HSP-47, and -70), cellular oxidative stress effects, and inflammatory cytokines [interleukin (IL)-1 and IL-8] in non-irradiated, UVA-radiated, UVB-radiated dermal fibroblasts. With regard to the ECM, vitamin D stimulated type I collagen and inhibited cellular elastase activity in non-irradiated fibroblasts; and stimulated type I collagen and HSP-47, and inhibited elastin expression and elastase activity in UVA-radiated dermal fibroblasts. With regard to cellular protection, vitamin D inhibited oxidative damage to DNA, RNA, and lipids in non-irradiated, UVA-radiated and UVB-radiated fibroblasts, and, in addition, increased cell viability of UVB-radiated cells. -

Proteins and Peptides As Important Modifiers of the Polymer Scaffolds
polymers Review Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review Katarzyna Klimek * and Grazyna Ginalska Chair and Department of Biochemistry and Biotechnology, Medical University of Lublin, Chodzki 1 Street, 20-093 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-81-448-7028; +48-81-448-7020 Received: 29 January 2020; Accepted: 2 April 2020; Published: 6 April 2020 Abstract: Polymer scaffolds constitute a very interesting strategy for tissue engineering. Even though they are generally non-toxic, in some cases, they may not provide suitable support for cell adhesion, proliferation, and differentiation, which decelerates tissue regeneration. To improve biological properties, scaffolds are frequently enriched with bioactive molecules, inter alia extracellular matrix proteins, adhesive peptides, growth factors, hormones, and cytokines. Although there are many papers describing synthesis and properties of polymer scaffolds enriched with proteins or peptides, few reviews comprehensively summarize these bioactive molecules. Thus, this review presents the current knowledge about the most important proteins and peptides used for modification of polymer scaffolds for tissue engineering. This paper also describes the influence of addition of proteins and peptides on physicochemical, mechanical, and biological properties of polymer scaffolds. Moreover, this article sums up the major applications of some biodegradable natural and synthetic polymer scaffolds modified with proteins and peptides, which have been developed within the past five years. Keywords: bioactive construct; biocompatibility; biomolecules; cytotoxicity; ECM; hydrogels; protein carrier; regenerative medicine; stem cells; tissue repair 1. Introduction: The Role of Proteins and Peptides in TE Tissue engineering (TE) is a multidisciplinary field, which constitutes an alternative and promising approach for grafts, i.e., autografts, allografts, and xenografts [1–3]. -

Elastin Biology and Tissue Engineering with Adult Cells
DOI 10.1515/bmc-2012-0040 BioMol Concepts 2013; 4(2): 173–185 Review Cassandra B. Saitow * , Steven G. Wise, Anthony S. Weiss , John J. Castellot Jr. and David L. Kaplan Elastin biology and tissue engineering with adult cells Abstract: The inability of adult cells to produce well-organ- collagen to allow for repeated cycles of expansion and ized, robust elastic fibers has long been a barrier to the contraction in response to pulsatile flow (3) . This review successful engineering of certain tissues. In this review, focuses primarily on blood vessel tissue engineering, we focus primarily on elastin with respect to tissue-engi- particularly the challenge of inducing sufficient elastin neered vascular substitutes. To understand elastin regu- expression from cells that colonize vascular constructs. lation during normal development, we describe the role Developmental regulation of elastin dictates that of various elastic fiber accessory proteins. Biochemical synthesis is predominantly in utero and early childhood pathways regulating expression of the elastin gene are (4) . New elastic fibers are not produced in appreciable addressed, with particular focus on tissue-engineering amounts under normal physiological circumstances in research using adult-derived cells. adult cells. This deficit presents a significant challenge in tissue engineering of vascular constructs that seek Keywords: elastin; heparin; smooth muscle cell; vascular to replicate the elastin content of natural vessels. This tissue engineering. review considers factors involved in elastic fiber forma- tion during normal development and addresses recent advances in the induction of elastin expression by cells *Corresponding author : Cassandra B. Saitow, Tufts University, from adult donors. Department of Biomedical Engineering, Medford, MA, 02155 , USA, e-mail: [email protected] Steven G. -

Extracellular Matrix Grafts: from Preparation to Application (Review)
INTERNATIONAL JOURNAL OF MOleCular meDICine 47: 463-474, 2021 Extracellular matrix grafts: From preparation to application (Review) YONGSHENG JIANG1*, RUI LI1,2*, CHUNCHAN HAN1 and LIJIANG HUANG1 1Science and Education Management Center, The Affiliated Xiangshan Hospital of Wenzhou Medical University, Ningbo, Zhejiang 315700; 2School of Chemistry, Sun Yat-sen University, Guangzhou, Guangdong 510275, P.R. China Received July 30, 2020; Accepted December 3, 2020 DOI: 10.3892/ijmm.2020.4818 Abstract. Recently, the increasing emergency of traffic acci- Contents dents and the unsatisfactory outcome of surgical intervention are driving research to seek a novel technology to repair trau- 1. Introduction matic soft tissue injury. From this perspective, decellularized 2. ECM-G characterization matrix grafts (ECM-G) including natural ECM materials, and 3. Methods of decellularization treatments their prepared hydrogels and bioscaffolds, have emerged as 4. Removal of residual cellular components and chemicals possible alternatives for tissue engineering and regenerative 5. Application of ECM-P in regenerative medicine medicine. Over the past decades, several physical and chemical 6. Challenges and future outlook on ECM-P decellularization methods have been used extensively to deal 7. Conclusions with different tissues/organs in an attempt to carefully remove cellular antigens while maintaining the non-immunogenic ECM components. It is anticipated that when the decellular- 1. Introduction ized biomaterials are seeded with cells in vitro or incorporated into irregularly shaped defects in vivo, they can provide the The extracellular matrix (ECM) derived from organs/tissues is appropriate biomechanical and biochemical conditions for a complex, highly organized assembly of macromolecules with directing cell behavior and tissue remodeling. -

Blood Vitronectin Is a Major Activator of LIF and IL-6 in the Brain Through Integrin–FAK and Upar Signaling Matthew P
© 2018. Published by The Company of Biologists Ltd | Journal of Cell Science (2018) 131, jcs202580. doi:10.1242/jcs.202580 RESEARCH ARTICLE Blood vitronectin is a major activator of LIF and IL-6 in the brain through integrin–FAK and uPAR signaling Matthew P. Keasey1, Cuihong Jia1, Lylyan F. Pimentel1,2, Richard R. Sante1, Chiharu Lovins1 and Theo Hagg1,* ABSTRACT Microglia and astrocytes express the VTN receptors αvβ3 and α β We defined how blood-derived vitronectin (VTN) rapidly and potently v 5 integrin (Herrera-Molina et al., 2012; Kang et al., 2008; activates leukemia inhibitory factor (LIF) and pro-inflammatory Milner, 2009; Welser-Alves et al., 2011). Microglia and astrocytes, interleukin 6 (IL-6) in vitro and after vascular injury in the brain. as well as endothelial cells, are major producers of pro- α in vitro Treatment with VTN (but not fibrinogen, fibronectin, laminin-111 or inflammatory cytokines, such as IL-6 and TNF , and collagen-I) substantially increased LIF and IL-6 within 4 h in after traumatic or ischemic injury to the brain (Banner et al., 1997; C6-astroglioma cells, while VTN−/− mouse plasma was less effective Erta et al., 2012; Lau and Yu, 2001) or upon self-induction by IL-6 than that from wild-type mice. LIF and IL-6 were induced by (Van Wagoner and Benveniste, 1999). IL-6 is a major regulator of a intracerebral injection of recombinant human (rh)VTN in mice, but variety of inflammatory disorders and a target for therapies (Hunter induction seen upon intracerebral hemorrhage was less in VTN−/− and Jones, 2015). -

With Caviar, Keratin & Collagen
WITH CAVIAR, KERATIN & COLLAGEN Professional treatments for colour, bleaching, care and maintenance with CAVIAR, KERATIN and COLLAGEN Professional styling products with CAVIAR, KERATIN and COLLAGEN Technical professional products with CAVIAR, KERATIN and COLLAGEN 2 Professional products by Very high technology professional products to colour, treat and protect hair from the continuous chemical and environmental stress caused on a daily basis. Formulas based on: Caviar Keratin Collagen 3 WITH CAVIAR, KERATIN & COLLAGEN Colouring permanentCream professional ammonia PPD • Respects the hair structure thanks to an exposure free free time of 12 minutes • Non-progressive • Ammonia free • Paraphenylenediamine free • Unleashes all the EFFICANCY of its active principles and maximum COLOURING POWER Mix 1 : 1 4 WITH CAVIAR, KERATIN & COLLAGEN 1. Respect for scalp and hair thanks to a shorter processing time 2. Maximum grey hair coverage 3. Lightens up to 4 tones 4. Ammonia free and Paraphenylenediamine free 5. Safe application even on customers with a sensitive scalp 6. Extreme colour brilliancy and uniformity 7. Very easy and practical to use 8. Long lasting reflections 9. High colour fastness 10. Great protection action 11. Effective restructuring action benefits 12. Maximum conditioning 5 WITH CAVIAR, KERATIN & COLLAGEN Benefits 1 2 3 Respect for scalp and hair thanks to a shorter processing Lightens up to Maximum grey hair 4 tones time coverage 6 WITH CAVIAR, KERATIN & COLLAGEN has been developed according Cosmetic colour pDT BASE Be Colour 12 Minute Dpe DIAMINOPHENOXYETHANOL to the rules of the “molar stoichiometry,” a technique creamy gel with RESORCINOL m-AMINOPHENOL of colouring clear and uncompromising. It is based on CAVIAR, a mathematical principle according to which the molar concentration of the dye base is equal to the molar KERATIN and concentration of the sum of the other colouring couplers. -

Incorporation of Recombinant Fibronectin Into Genetically Engineered Elastin-Based Polymers
Incorporation of Recombinant Fibronectin Into Genetically Engineered Elastin-based Polymers A Thesis Presented to The Academic Faculty by Fanor Alberto Balderrama In Partial Fulfillment of the Requirements for the Degree Master of Science in the School of Bioengineering Georgia Institute of Technology December 2009 Incorporation of Recombinant Fibronectin Into Genetically Engineered Elastin-based Polymers Approved by: Dr. Elliot L. Chaikof, Advisor School of Biomedical Engineering Georgia Institute of Technology Department of Surgery Emory University School of Medicine Dr. Hanjoong Jo School of Biomedical Engineering Georgia Institute of Technology Division of Cardiology Emory University School of Medicine Dr. Vincent P. Conticello School of Chemistry Emory University Date Approved: November 11, 2009 ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. Elliot Chaikof, for the opportunity of working on this research and for all the guidance. I would also like to thank Dr. Carolyn Haller for all her priceless guidance, her supervision and patience, without which this thesis would have not been possible. My sincere appreciation goes out to members of my committee: Dr. Hanjoong Jo and Dr. Vincent Conticello for their help with the direction and the review of this thesis. I would also like to thank all the other members of the Chaikof laboratory for their friendship, their help and their advice, without which my research experience would have not been so enjoyable. Last, but absolutely not least, I thank my family for all their support (professional, emotional and even financial) and their faith in me. iii TABLE OF CONTENTS Acknowledgements iii List of figures vii List of abbreviations viii Summary x Chapter I: Introduction 1 1.1. -
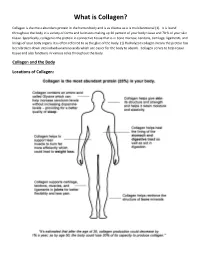
What Is Collagen?
What is Collagen? Collagen is the most abundant protein in the human body and is as diverse as it is multifunctional (1). It is found throughout the body in a variety of forms and functions making up 30 percent of your body tissue and 70 % of your skin tissue. Specifically, collagen is the protein in connective tissue that is in bone marrow, tendons, cartilage, ligaments, and linings of your body organs. It is often referred to as the glue of the body. (1) Hydrolyzed collagen means the protein has been broken down into individual amino acids which are easier for the body to absorb. Collagen serves to help repair tissue and also functions in various roles throughout the body. Collagen and the Body Locations of Collagen: What Does Collagen Do? Collagen has numerous structural properties but also plays a vital role in the repair of almost all the body’s tissues. Some diseases are directly linked to lacking this essential protein. Depending on which part of the body it is located, collagen serves different purposes. In skin: Found in the inner layer, this connective tissue gives the skin its structure and strength and also functions in the replacement of dead skin cells. A lack of collagen in the skin can contribute to a decrease in skin health leading to stretch marks, dark spots, and infections as well as affecting the skin’s ability to maintain moisture. In internal organs and blood vessels: In the lining of your organs like in the stomach, kidneys, blood vessels and spleen, collagen functions as a protective covering and a fibrous barrier.